��Ŀ����
ú�������Ǹ�Ч����������ú̿����Ҫ;��֮һ��
(1)��250C 101kPaʱ��H2��O2��������1mol H2O(g)�ų�241.8kJ�����������Ȼ�ѧ����ʽΪ
___________
��֪: ��C(s)��O2(g)�TCO2(g) ��H����393.5kJ/mol
��CO(g)�� O2(g)�TCO2(g) ��H����283.0kJ/mol
O2(g)�TCO2(g) ��H����283.0kJ/mol
��̿��ˮ������Ӧ�ǽ�����ú��Ϊ����ȼ�ϵķ�����C(s)��H2O(g)�TCO(g)��H2(g) ��H=____kJ/mol
(2) CO������H2O(g)��һ��������Ӧ: CO(g)��H2O(g) CO2(g)��H2(g) ��H��0�ں����ܱ������У���ʼʱn(H2O)=0.20mol��n(CO)��0.10 mol,��8000Cʱ�ﵽƽ��״̬��K��1.0����ƽ��ʱ��������CO��ת������_____________(����������һλС��)��
CO2(g)��H2(g) ��H��0�ں����ܱ������У���ʼʱn(H2O)=0.20mol��n(CO)��0.10 mol,��8000Cʱ�ﵽƽ��״̬��K��1.0����ƽ��ʱ��������CO��ת������_____________(����������һλС��)��
(3) ��ҵ�ϴ�ú������Ļ�����з����H2�����а��ĺϳɣ���֪��Ӧ��ӦN2(g)��3H2(g 2NH3(g)����H��0���ڵ��������½��У��ı�������Ӧ��������I��II��III����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��
2NH3(g)����H��0���ڵ��������½��У��ı�������Ӧ��������I��II��III����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��

��N2��ƽ����Ӧ����v1(N2)��vII(N2)��vIII(N2)�Ӵ�С���д���Ϊ________��
���ɵ�һ��ƽ��ڶ���ƽ�⣬ƽ���ƶ��ķ��� ��________����ȡ�Ĵ�ʩ��________��
�۱Ƚϵ�II�η�Ӧ�¶�(T2)�͵�III�η�Ӧ�ٶȣ�T3)�ĸߵͣ�T2________T3�����=��<���жϵ�������________________��
(1)��250C 101kPaʱ��H2��O2��������1mol H2O(g)�ų�241.8kJ�����������Ȼ�ѧ����ʽΪ
___________
��֪: ��C(s)��O2(g)�TCO2(g) ��H����393.5kJ/mol
��CO(g)��
 O2(g)�TCO2(g) ��H����283.0kJ/mol
O2(g)�TCO2(g) ��H����283.0kJ/mol��̿��ˮ������Ӧ�ǽ�����ú��Ϊ����ȼ�ϵķ�����C(s)��H2O(g)�TCO(g)��H2(g) ��H=____kJ/mol
(2) CO������H2O(g)��һ��������Ӧ: CO(g)��H2O(g)
 CO2(g)��H2(g) ��H��0�ں����ܱ������У���ʼʱn(H2O)=0.20mol��n(CO)��0.10 mol,��8000Cʱ�ﵽƽ��״̬��K��1.0����ƽ��ʱ��������CO��ת������_____________(����������һλС��)��
CO2(g)��H2(g) ��H��0�ں����ܱ������У���ʼʱn(H2O)=0.20mol��n(CO)��0.10 mol,��8000Cʱ�ﵽƽ��״̬��K��1.0����ƽ��ʱ��������CO��ת������_____________(����������һλС��)��(3) ��ҵ�ϴ�ú������Ļ�����з����H2�����а��ĺϳɣ���֪��Ӧ��ӦN2(g)��3H2(g
 2NH3(g)����H��0���ڵ��������½��У��ı�������Ӧ��������I��II��III����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��
2NH3(g)����H��0���ڵ��������½��У��ı�������Ӧ��������I��II��III����ϵ�и�����Ũ����ʱ��仯����������ͼ��ʾ��
��N2��ƽ����Ӧ����v1(N2)��vII(N2)��vIII(N2)�Ӵ�С���д���Ϊ________��
���ɵ�һ��ƽ��ڶ���ƽ�⣬ƽ���ƶ��ķ��� ��________����ȡ�Ĵ�ʩ��________��
�۱Ƚϵ�II�η�Ӧ�¶�(T2)�͵�III�η�Ӧ�ٶȣ�T3)�ĸߵͣ�T2________T3�����=��<���жϵ�������________________��
��14�֣���1��H2(g)+ O2(g)�TlH2O(g) ��H����241.8kJ/mol ��2�֣�����131.3kJ ��1�֣�
O2(g)�TlH2O(g) ��H����241.8kJ/mol ��2�֣�����131.3kJ ��1�֣�
��2��66.7% ��2�֣� ��3����v1(N2)��vII(N2)��vIII(N2)��3�֣�
��������Ӧ���� �ӷ�Ӧ��ϵ���Ƴ�����NH3��3�֣�
�ۣ� �˷�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ��������Ӧ�����ƶ� ��3�֣�
 O2(g)�TlH2O(g) ��H����241.8kJ/mol ��2�֣�����131.3kJ ��1�֣�
O2(g)�TlH2O(g) ��H����241.8kJ/mol ��2�֣�����131.3kJ ��1�֣���2��66.7% ��2�֣� ��3����v1(N2)��vII(N2)��vIII(N2)��3�֣�
��������Ӧ���� �ӷ�Ӧ��ϵ���Ƴ�����NH3��3�֣�
�ۣ� �˷�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ��������Ӧ�����ƶ� ��3�֣�
�����������1����25�桢101kPaʱ��H2��O2��������1molH2O(g)�ų�241.8kJ���������������Ȼ�ѧ����ʽΪH2(g)+
 O2(g)�TlH2O(g) ��H����241.8kJ/mol��
O2(g)�TlH2O(g) ��H����241.8kJ/mol����֪��C(s)��O2(g)�TCO2(g) ��H����393.5kJ/mol
��CO(g)��
 O2(g)�TCO2(g) ��H����283.0kJ/mol
O2(g)�TCO2(g) ��H����283.0kJ/mol��H2(g)+
 O2(g)�TlH2O(g) ��H����241.8kJ/mol
O2(g)�TlH2O(g) ��H����241.8kJ/mol���Ը��ݸ�˹���ɣ��٣��ۣ��ڼ��õ�C(s)��H2O(g)�TCO(g)��H2(g)����Ӧ�ȡ�H����393.5kJ/mol��241.8kJ/mol��283.0kJ/mol����131.3kJ/mol��
��2�� CO(g)��H2O(g)
 CO2(g)��H2(g)
CO2(g)��H2(g)��ʼ����mol�� 0.1 0.2 0 0
ת������mol�� x x x x
ƽ������mol�� 0.1��x 0.2��x x x
���ڷ�Ӧǰ�������������䣬���������ʵ�������Ũ�ȼ���ƽ�ⳣ��
��
 ��1.0
��1.0���x��

����ƽ��ʱ��������CO��ת������
 ��100%��66.7%
��100%��66.7%��3���ٸ���ͼ���֪�����߱�ʾ������Ũ�ȱ仯����v1(N2)����2mol/L��1mol/L����20min��0.05mol/��L?min����vII(N2)����1mol/L��0.62mol/L����15min��0.0253mol/��L?min����vIII(N2)����0.62mol/L��0.5mol/L����10min��0.012mol/��L?min������N2��ƽ����Ӧ����v1(N2)��vII(N2)��vIII(N2).
�ڸ���ͼ���֪���ڢ�ΰ����Ǵ�0��ʼ�ģ�˲�䷴Ӧ�ﵪ��������Ũ�Ȳ��䣬��˿���ȷ����һ��ƽ������ϵ���Ƴ��˰�����������������Ũ�ȣ�ƽ�������ƶ���
�۵ڢ�εĿ�ʼ��ڢ�ε�ƽ������ʵ�������ȣ����ݰ����������������٣������������ӿ��ж�ƽ���������ƶ��ġ�����ƽ�ʼʱŨ��ȷ����ƽ���ƶ�����������Ũ�ȵı仯����ģ�������Ŀ��������������������䣬��ı�ѹǿҲ�����ܣ����һ��Ϊ�¶ȵ�Ӱ�졣�˷�Ӧ����Ϊ���ȷ�Ӧ�������Ʋ�Ϊ�����¶ȣ���˴ﵽƽ����¶�һ���ȵڢ��ƽ��ʱ���¶ȵͣ���T2��T3��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 CH3OH��H2O����ü״������۲����뷴Ӧ�¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ����ش��������⣺
CH3OH��H2O����ü״������۲����뷴Ӧ�¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ����ش��������⣺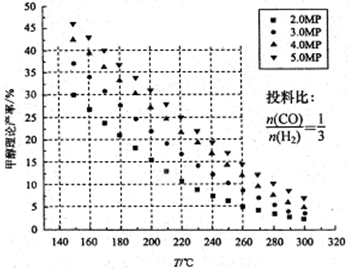
 ��
�� ��һ283.0 kJ
��һ283.0 kJ ��
�� ������300�棩��
������300�棩��
 ʱ���ӷ�Ӧ��ʼ����Ӧ�ﵽƽ�⣬���ɼ״���ƽ������Ϊ��
ʱ���ӷ�Ӧ��ʼ����Ӧ�ﵽƽ�⣬���ɼ״���ƽ������Ϊ��
 ����
����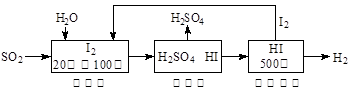
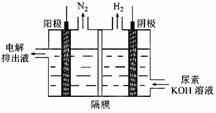
 Ni(OH)2��M
Ni(OH)2��M 4NO(g)+6H2O(g) ��H =" ��905" kJ/mol ������Ӧ
4NO(g)+6H2O(g) ��H =" ��905" kJ/mol ������Ӧ
 N2(g)��CO2(g)ij�о�С��������ܱ���������һ�����Ļ���̿��NO�����£�T��C)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ������
N2(g)��CO2(g)ij�о�С��������ܱ���������һ�����Ļ���̿��NO�����£�T��C)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ������
 N2��2CO2 ��H<0�о���������ʹ�õ���������ʱ����������ıȱ���������ѧ��Ӧ���ʣ�Ϊ�˷ֱ���֤�¶ȡ������ıȱ�����Ի�ѧ�� Ӧ���ʵ�Ӱ����ɡ�ijͬѧ���������ʵ�飬����ʵ�������Ѿ������±��С�
N2��2CO2 ��H<0�о���������ʹ�õ���������ʱ����������ıȱ���������ѧ��Ӧ���ʣ�Ϊ�˷ֱ���֤�¶ȡ������ıȱ�����Ի�ѧ�� Ӧ���ʵ�Ӱ����ɡ�ijͬѧ���������ʵ�飬����ʵ�������Ѿ������±��С�
