��Ŀ����
��2014��ӱ�ʡ��ɽ�и�����һ��ģ�⿼�����ۻ�ѧ�Ծ���
�����ڹ�ũҵ����������Ҫ����;��ijУ�ס���������ѧС��ֱ�������ʵ��������о���

��1���ס�����С��ѡ���˲�ͬ������ȡ������
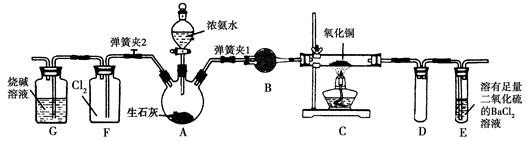
�뽫ʵ��װ�õ���ĸ��ź��Ʊ�ԭ����д���±��ո��С�
��2������ͨ����ͼװ�ã�β������װ������ȥ����֤�˰��л�ԭ�ԣ�װ���е�ʵ��������____ ��Ӳ�ʲ������а�������������Ӧ�Ļ�ѧ����ʽΪ ��

��3�����齫������������ͨ����з�̪��AgNO3��Һ�У�����Ϊ ��
��Ӧ����������Һ�г���H+��OH����NH4+���Ҫ���е������� ��
��4���������Ʊ�����þ���������Ϸ��ְ�������ͭ��Ӧ�����Ʊ�������2NH3+3CuO 3Cu+N2+3H2O������������þ�ڸ����·�Ӧ�ɵõ�����þ��������þ��ˮ������Ӧ����Mg��OH��2��NH3����������������Ʊ�����þ��ʵ�鷽��ʾ���ͼ��ʵ��ǰϵͳ�ڿ������ų���ͼ�м�ͷ��ʾ�������������Ϊ�˷����Ƿ���ȷ����˵������ ��
3Cu+N2+3H2O������������þ�ڸ����·�Ӧ�ɵõ�����þ��������þ��ˮ������Ӧ����Mg��OH��2��NH3����������������Ʊ�����þ��ʵ�鷽��ʾ���ͼ��ʵ��ǰϵͳ�ڿ������ų���ͼ�м�ͷ��ʾ�������������Ϊ�˷����Ƿ���ȷ����˵������ ��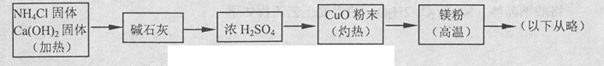
�����ڹ�ũҵ����������Ҫ����;��ijУ�ס���������ѧС��ֱ�������ʵ��������о���

��1���ס�����С��ѡ���˲�ͬ������ȡ������
�뽫ʵ��װ�õ���ĸ��ź��Ʊ�ԭ����д���±��ո��С�
| | ʵ��װ�� | ʵ��ҩƷ | �Ʊ�ԭ�� |
| ��С�� | A | �������ơ��Ȼ�� | ��Ӧ�Ļ�ѧ����ʽΪ �� |
| ��С�� | �� | Ũ��ˮ���������� | �û�ѧƽ��ԭ�������������Ƶ����ã� �� |
��2������ͨ����ͼװ�ã�β������װ������ȥ����֤�˰��л�ԭ�ԣ�װ���е�ʵ��������____ ��Ӳ�ʲ������а�������������Ӧ�Ļ�ѧ����ʽΪ ��

��3�����齫������������ͨ����з�̪��AgNO3��Һ�У�����Ϊ ��
��Ӧ����������Һ�г���H+��OH����NH4+���Ҫ���е������� ��
��4���������Ʊ�����þ���������Ϸ��ְ�������ͭ��Ӧ�����Ʊ�������2NH3+3CuO
 3Cu+N2+3H2O������������þ�ڸ����·�Ӧ�ɵõ�����þ��������þ��ˮ������Ӧ����Mg��OH��2��NH3����������������Ʊ�����þ��ʵ�鷽��ʾ���ͼ��ʵ��ǰϵͳ�ڿ������ų���ͼ�м�ͷ��ʾ�������������Ϊ�˷����Ƿ���ȷ����˵������ ��
3Cu+N2+3H2O������������þ�ڸ����·�Ӧ�ɵõ�����þ��������þ��ˮ������Ӧ����Mg��OH��2��NH3����������������Ʊ�����þ��ʵ�鷽��ʾ���ͼ��ʵ��ǰϵͳ�ڿ������ų���ͼ�м�ͷ��ʾ�������������Ϊ�˷����Ƿ���ȷ����˵������ ��
��1���� 2NH4Cl��Ca��OH��2 CaCl2��2H2O��2NH3������2�֣���B ��1�֣�
CaCl2��2H2O��2NH3������2�֣���B ��1�֣�
�۹���NaOH����Ũ��ˮ�ų��������ȣ���ʹNH3�Ļӷ�����Һ��OH��Ũ�ȵ����ӣ�ʹNH3��H2O NH3��H2O
NH3��H2O NH4�� ��OH��������NH3�ķ����ƶ�����2�֣�
NH4�� ��OH��������NH3�ķ����ƶ�����2�֣�
��2���������г��ֺ���ɫ��������й��������2�֣� ��2�֣�
��2�֣�
��3���Ȳ�����ɫ����������ʧ����Һ����ɺ�ɫ ��2�֣�Ag��NH3��2+��NO3�� ��2�֣�
��4����������Ӧ������NH3ȫ����H2SO4����,���ܽ��к�����Ӧ����2�֣�
 CaCl2��2H2O��2NH3������2�֣���B ��1�֣�
CaCl2��2H2O��2NH3������2�֣���B ��1�֣��۹���NaOH����Ũ��ˮ�ų��������ȣ���ʹNH3�Ļӷ�����Һ��OH��Ũ�ȵ����ӣ�ʹNH3��H2O
 NH3��H2O
NH3��H2O NH4�� ��OH��������NH3�ķ����ƶ�����2�֣�
NH4�� ��OH��������NH3�ķ����ƶ�����2�֣���2���������г��ֺ���ɫ��������й��������2�֣�
 ��2�֣�
��2�֣���3���Ȳ�����ɫ����������ʧ����Һ����ɺ�ɫ ��2�֣�Ag��NH3��2+��NO3�� ��2�֣�
��4����������Ӧ������NH3ȫ����H2SO4����,���ܽ��к�����Ӧ����2�֣�
��1�����������ơ��Ȼ����ȡ�����Ļ�ѧ����ʽΪ2NH4Cl��Ca��OH��2
 CaCl2��2H2O��2NH3��
CaCl2��2H2O��2NH3����Ũ��ˮ������������ȡ����Ӧѡ��Bװ�ã�
�۰�ˮ�д���NH3��H2O
 NH3��H2O
NH3��H2O NH4�� ��OH����ƽ�⣬����NaOH���壬һ��OH��Ũ������ƽ�������ƶ�������NaOH��������ˮ��ų��������ȣ���ʹNH3�Ļӷ���
NH4�� ��OH����ƽ�⣬����NaOH���壬һ��OH��Ũ������ƽ�������ƶ�������NaOH��������ˮ��ų��������ȣ���ʹNH3�Ļӷ�����2�������������ڴ�������������һ���������壬��������������Ϊ����ɫ����������ͬʱ����ˮ���ɣ�������ˮ����ͭ����ɫ��
��3�����齫������������ͨ����з�̪��AgNO3��Һ�У���������ˮ����Һ�Լ��ԣ���Ag+��Ӧ����������������������ͨ�����������������������������ܽ⣬������Һ���ɫ����Ӧ����������Һ�г���H+��OH����NH4+���Ҫ���е�����Ag��NH3��2+��NO3��
��4������ȷ����Ӧ�����İ���ȫ����Ũ�������գ������к���ķ�Ӧ

��ϰ��ϵ�д�
�����Ŀ