��Ŀ����
| |||||||||||||||||||||||||
�𰸣�3��C;
������
��ʾ��
������
(1) |
|
(2) |
����������Cl2����ʴ�ѣ��ܷ�ֹH2��Cl2��ϱ�ը�����ܱ���Cl2��NaOH��Ӧ |
(3) |
���ʱH+��Cl���ֱ��������������ϵ�ʧ���ӣ�����H2��Cl2���������ϱ���ѡ���ױ�Cl2��ʴ�IJ��ϣ��������������¶ȡ������ѹ�������ڵ�����ʵļӿ죮 |
��ʾ��
|
��Ϥ���ԭ����Ĥ�����ú�������ѡ�� |

��ϰ��ϵ�д�
�����Ŀ
 ��ʽ̼��ͭ Cu2��OH��2CO3��һ����;�㷺�Ļ���ԭ�ϣ�ʵ�����Է�ͭмΪԭ����ȡ��ʽ̼��ͭ�IJ������£�
��ʽ̼��ͭ Cu2��OH��2CO3��һ����;�㷺�Ļ���ԭ�ϣ�ʵ�����Է�ͭмΪԭ����ȡ��ʽ̼��ͭ�IJ������£�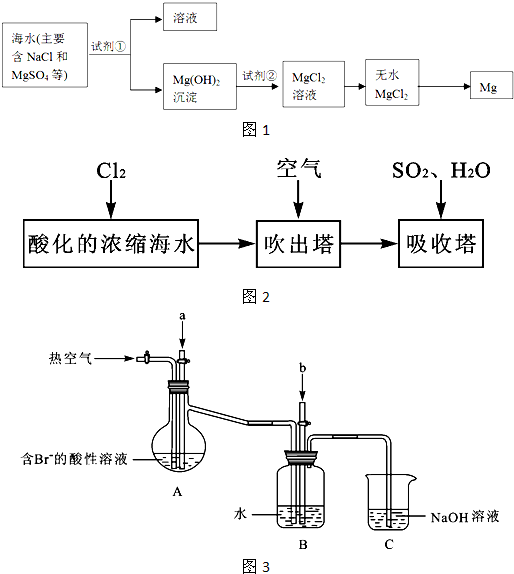
 ���������գ�
���������գ�
