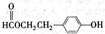��Ŀ����
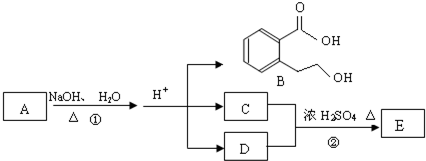
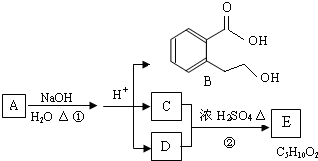
A��B��C��D��E��Ϊ�л������A�Ƿ���ʽΪC5H10O��ֱ�������B��NaHCO3��Һ��ȫ��Ӧ�������ʵ���֮��Ϊ1��2������֮��Ĺ�ϵ��ͼ��ʾ����ʾ��RCH=CHR�������Ը��������Һ�з�Ӧ����RCOOH��R��COOH������R��R��Ϊ���������������������ǣ�������

| A��B�Ľṹ��ʽΪHOOC-CH2-COOH | B��C���Ҵ�������������Ӧ | C��A����������������Һ�����кͷ�Ӧ | D��E�ķ���ʽΪC3H2O4Na2 |
������A�ķ���ʽΪC5H10O�������Ͷ�Ϊ
=1�������Ը��������������B��C������Ϣ��֪��A����1��C=C˫����B��C�������Ȼ���C���Ҵ�����������Ӧ����DΪC4H8O2����CΪCH3COOH��DΪCH3COOC2H5��B��̼ԭ����ĿΪ3������̼�����ư�1��2��Ӧ����B�����к���2���Ȼ�-COOH��˵��A�����л�����1��-OH����A�Ľṹ��ʽΪCH3CH=CHCH2CH2OH����BΪHOOCCH2COOH����̼�����Ʒ�Ӧ����EΪNaOOCCH2COONa���ݴ˽��
| 2��5+2-10 |
| 2 |
����⣺A�ķ���ʽΪC5H10O�������Ͷ�Ϊ
=1�������Ը��������������B��C������Ϣ��֪��A����1��C=C˫����B��C�������Ȼ���C���Ҵ�����������Ӧ����DΪC4H8O2����CΪCH3COOH��DΪCH3COOC2H5��B��̼ԭ����ĿΪ3������̼�����ư�1��2��Ӧ����B�����к���2���Ȼ�-COOH��˵��A�����л�����1��-OH����A�Ľṹ��ʽΪCH3CH=CHCH2CH2OH����BΪHOOCCH2COOH����̼�����Ʒ�Ӧ����EΪNaOOCCH2COONa��
A��������������֪��BΪHOOCCH2COOH����A��ȷ��
B��C�����Ȼ������Ҵ�����������Ӧ����B��ȷ��
C��AΪCH3CH=CHCH2CH2OH���������������Ʒ�Ӧ����C����
D��EΪNaOOCCH2COONa������ʽΪC3H2O4Na2����D��ȷ��
��ѡC��
| 2��5+2-10 |
| 2 |
A��������������֪��BΪHOOCCH2COOH����A��ȷ��
B��C�����Ȼ������Ҵ�����������Ӧ����B��ȷ��
C��AΪCH3CH=CHCH2CH2OH���������������Ʒ�Ӧ����C����
D��EΪNaOOCCH2COONa������ʽΪC3H2O4Na2����D��ȷ��
��ѡC��
���������⿼���л�����ƶϣ���Ҫѧ���Ը������Ϣ�������ã�ע�����չ����ŵ�������ת�����ۺϿ���ѧ�������������������Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ