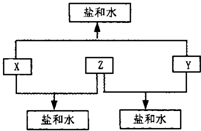
��Ŀ����
��֪����A��B��C��D�������ʾ�����Ԫ��X�����ܻ�����Ԫ��Y��Z��Ԫ��Y��X��Z��ԭ���������ε����Ҳ�����20����X��A��B��C��D�еĻ��ϼ۷ֱ�Ϊ0��-1��+1��+5���������µ���A��ij�ֳ���һԪǿ����Һ��Ӧ���ɵõ�B��C���ܻ�����D�ڶ������̴������ȷֽ⣬���Ƶ�Ԫ��Y�ĵ��ʣ���ش��������⣺
��1��д��Ԫ��X��Y��Z��Ԫ�ط��ţ�X��______��Y��______��Z______��
��2��д��A��B��C��D�Ļ�ѧʽ��A______��B______��C______��D______��
��3������Yԭ�ӵĽṹʾ��ͼ______��д�����з�Ӧ�Ļ�ѧ����ʽ______��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ______��ʵ����ͨ����Ӧ���Ƶ�Ԫ��Y�ĵ��ʺ���ν�������벢�ֱ��ô����д��ʵ�鲽�裺
��5���Ƶ�Ԫ��Y�ĵ��ʣ����ռ�����״����6.72LY�������壬������Ҫ���ٿ˻�����D��ͬʱ�����Ȼ�������ʵ���Ϊ����mol�������漰���Ļ�ѧʽд�����㲽�裩
��1��д��Ԫ��X��Y��Z��Ԫ�ط��ţ�X��______��Y��______��Z______��
��2��д��A��B��C��D�Ļ�ѧʽ��A______��B______��C______��D______��
��3������Yԭ�ӵĽṹʾ��ͼ______��д�����з�Ӧ�Ļ�ѧ����ʽ______��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ______��ʵ����ͨ����Ӧ���Ƶ�Ԫ��Y�ĵ��ʺ���ν�������벢�ֱ��ô����д��ʵ�鲽�裺
��5���Ƶ�Ԫ��Y�ĵ��ʣ����ռ�����״����6.72LY�������壬������Ҫ���ٿ˻�����D��ͬʱ�����Ȼ�������ʵ���Ϊ����mol�������漰���Ļ�ѧʽд�����㲽�裩
��1��������D�ڶ������̴������ȷֽ⣬���Ƶ�Ԫ��Y�ĵ��ʿ�֪DΪKClO3��YΪO����X��A��B��C��D�еĻ��ϼ۷ֱ�Ϊ0��-1��+1��+5��-1�۵Ķ�����Ԫ�����б�۵�Ԫ��XΪCl��Ԫ��Y��X��Z��ԭ���������ε����Ҳ�����20��ZΪK���ʴ�Ϊ��Cl��O��K��
��2������AΪCl2��Cl2��ij�ֳ���һԪǿ����Һ��Ӧ���ɵõ�BNaCl��CNaClO��������D�ڶ������̴������ȷֽ⣬���Ƶ�Ԫ��Y�ĵ��ʿ�֪DΪKClO3��
�ʴ�Ϊ��Cl2 ��NaCl��NaClO��KClO3��
��3����ԭ�������6�����ӣ�ԭ�ӽṹʾ��ͼΪ

��Cl2��NaOH��Ӧ����NaCl��NaClO��H2O��
�ʴ�Ϊ��

��Cl2+2NaOH=NaCl+NaClO+H2O��
��4��KClO3�ڶ������̴������ȷֽ⣬����Ӧ��Ļ��������ˮ����ˣ��ʴ�Ϊ��2KClO3
2KCl+3O2 ����
����Ӧ��Ļ����������̣�KCl����ˮ����ˣ��������̲�����ˮΪ������KCl������ˮΪ��Һ������Һ�����ᾧ�ɵ�KCl��
��5��n��O2 ��=
=0.3moL��
����ҪX�˻�����KClO3��ͬʱ�����Ȼ�������ʵ���ΪYmol
2KClO3
2KCl+3O2 ��
122.5g 2moL 3mol
X Y 0.3mol
=
=
��ã�X=12.25g��Y=0.2mol��
����Ҫ12.25g������KClO3��ͬʱ�����Ȼ�������ʵ���Ϊ0.2mol��
��2������AΪCl2��Cl2��ij�ֳ���һԪǿ����Һ��Ӧ���ɵõ�BNaCl��CNaClO��������D�ڶ������̴������ȷֽ⣬���Ƶ�Ԫ��Y�ĵ��ʿ�֪DΪKClO3��
�ʴ�Ϊ��Cl2 ��NaCl��NaClO��KClO3��
��3����ԭ�������6�����ӣ�ԭ�ӽṹʾ��ͼΪ

��Cl2��NaOH��Ӧ����NaCl��NaClO��H2O��
�ʴ�Ϊ��

��Cl2+2NaOH=NaCl+NaClO+H2O��
��4��KClO3�ڶ������̴������ȷֽ⣬����Ӧ��Ļ��������ˮ����ˣ��ʴ�Ϊ��2KClO3
| ||
| �� |
����Ӧ��Ļ����������̣�KCl����ˮ����ˣ��������̲�����ˮΪ������KCl������ˮΪ��Һ������Һ�����ᾧ�ɵ�KCl��
��5��n��O2 ��=
| 6.72L |
| 22.4moL/L |
����ҪX�˻�����KClO3��ͬʱ�����Ȼ�������ʵ���ΪYmol
2KClO3
| ||
| �� |
122.5g 2moL 3mol
X Y 0.3mol
| 122.5g |
| X |
| 3moL |
| 0.3moL |
| 2moL |
| Y |
��ã�X=12.25g��Y=0.2mol��
����Ҫ12.25g������KClO3��ͬʱ�����Ȼ�������ʵ���Ϊ0.2mol��

��ϰ��ϵ�д�
�����Ŀ
H2��I2��һ���������ܷ�����Ӧ��H2��g��+I2��g��?2HI��g����H=-a kJ?mol-1
��֪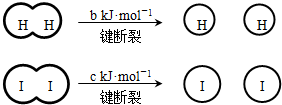 ��a��b��c�������㣩
��a��b��c�������㣩
����˵������ȷ���ǣ�������
��֪
 ��a��b��c�������㣩
��a��b��c�������㣩����˵������ȷ���ǣ�������
| A����Ӧ�������������������������� | B���Ͽ�1mol H-H����1mol I-I�������������ڶϿ�2mol H-I���������� | C���Ͽ�2mol H-I����������ԼΪ��c+b+a��kJ | D�����ܱ������м���2mol H2��2mol I2����ַ�Ӧ��ų�������С��2a kJ |

 ��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D2-������E2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��
��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D2-������E2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��
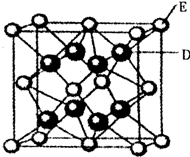 ��֪��A��B��C��D��E��F�����ڱ���ǰ36��Ԫ�أ�A��ԭ�Ӱ뾶��С��Ԫ�أ�BԪ�ػ�̬ԭ�ӵ�2P�����ֻ���������ӣ�CԪ�صĻ�̬ԭ��L��ֻ��2�ԳɶԵ��ӣ�D��Ԫ�����ڱ��е縺������Ԫ�أ�E2+�ĺ�������Ų���Arԭ����ͬ��F�ĺ˵������D��E�ĺ˵����֮�ͣ�
��֪��A��B��C��D��E��F�����ڱ���ǰ36��Ԫ�أ�A��ԭ�Ӱ뾶��С��Ԫ�أ�BԪ�ػ�̬ԭ�ӵ�2P�����ֻ���������ӣ�CԪ�صĻ�̬ԭ��L��ֻ��2�ԳɶԵ��ӣ�D��Ԫ�����ڱ��е縺������Ԫ�أ�E2+�ĺ�������Ų���Arԭ����ͬ��F�ĺ˵������D��E�ĺ˵����֮�ͣ�