МвДїДЪИЭ
AКЗµЄЎўЗвБЅЦЦФЄЛШЧйіЙµД»ЇєПОпЈ¬Ул№эСх»ЇЗвєНЛ®Ц®јдФЪЅб№№ЙПµД№ШПµПаЛЖЈ¬°±·ЦЧУЦРТ»ЦЦє¬µЄµД»щНЕФЪA·ЦЧУЦРѕЯУРБЅёцЈ¬ЗлМоРґТФПВїХ°ЧЎЈ(1)AµД·ЦЧУКЅКЗ___ЎЎЎЎ ____ЎЈ
(2)AµДЛ®ИЬТєіК_______(МоЛбЎўјоЎўЦР)РФЈ¬1 mol AЧо¶аїЙУл_______mol HCl(»тNaOH)·ўЙъЦРєН·ґУ¦РОіЙХэСОЈ¬РґіцРОіЙµДСОЦРє¬УРµЄФЧУµДАлЧУµДµзЧУКЅ___ЎЎЎЎЎЎЎЎ ____ЎЈ
(3)AФЪСх»Ї»№Ф·ґУ¦ЦРТІУл№эСх»ЇЗвПаЛЖЈ¬јИїЙЧчСх»ЇјБЈ¬УЦїЙЧч»№ФјБЎЈЗлёщѕЭјЫМ¬·ЦОцІъЙъХвЦЦЗйїцµДФТт____ЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎЎ ___ЎЈ
ЅвОцЈє
(1)N2H4ЎЈ(2)јоРФЈ»2mol HClЈ»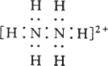 ЎЈ ЎЈ
(3)N2H4ЦРµЄµД»ЇєПјЫОЄ-2јЫЈ¬їЙТФЙэёЯТІїЙТФЅµµНЈ¬№КN2H4ФЪСх»Ї»№Ф·ґУ¦ЦРјИїЙЧчСх»ЇјБУЦїЙЧч»№ФјБЎЈ
|
МбКѕЈє
H2OєНH2O2Ц®јдЈ¬Ѕб№№ЙПїґH2O·ЦЧУЦРОЮ№эСхјь(OЎЄO)ЎЈ¶шNH3ЦРОЮNЎЄNµҐјьЈ¬№КAµДЅб№№У¦ОЄ )Ј¬ТЧµГ2ёцЦКЧУ(H+)РОіЙNH )Ј¬ТЧµГ2ёцЦКЧУ(H+)РОіЙNH ЎЈN2H4ЦРµЄµД»ЇєПјЫОЄ-2јЫЈ¬УлH2O2ЦРСхТ»СщЈ¬µЄµД»ЇєПјЫјИїЙЙэёЯУЦїЙЅµµНЈ¬№КФЪ»ЇС§·ґУ¦ЦРN2H4їЙЧчСх»ЇјБУЦїЙЧч»№ФјБЎЈ ЎЈN2H4ЦРµЄµД»ЇєПјЫОЄ-2јЫЈ¬УлH2O2ЦРСхТ»СщЈ¬µЄµД»ЇєПјЫјИїЙЙэёЯУЦїЙЅµµНЈ¬№КФЪ»ЇС§·ґУ¦ЦРN2H4їЙЧчСх»ЇјБУЦїЙЧч»№ФјБЎЈ
|

| |||||||||||||||