��Ŀ����
��ȸʯ����Ҫ�ɷ�ΪCu2(OH)2CO3���������������������������������ʵ
�����Կ�ȸʯΪԭ���Ʊ�CuSO4��5H2O���������ͼ��
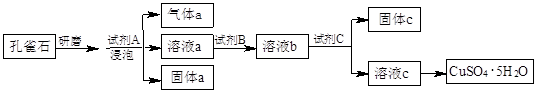
��1����ĥ��ȸʯ��Ŀ���� �����ݿ�ȸʯ���Լ�A��ѡ�ù�����ϡ���ᣬ�����a�� (�ѧʽ)��
��2���Լ�B��Ŀ���ǽ���Һ�е�Fe2+ת��ΪFe3+�����Լ�B��ѡ�� ������ţ���
A������KMnO4��Һ B��˫��ˮ C��Ũ���� D����ˮ
��Ӧ�����ӷ���ʽΪ�� ��
��3���Լ�C���ڵ�����ҺpH��ʹFe3+ת��Ϊ�������Է��롣���Լ�C��ѡ�� ������ţ���
A��ϡ���� B��NaOH��Һ C����ˮ D��CuO
����C�Ļ�ѧʽΪ ��
��4��1 mol����ͨ�����ȵ�Cu2(OH)2 CO3���Բ���1.5 mol����ͭ����÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��������Fe(OH)3��Ksp=1��10-39����Ҫ����Һ�е�Fe3+ת��ΪFe(OH)3������ʹ��Һ��c(Fe3+)������1��10��3 mol��L�����轫��Һ������pH= ��
�����Կ�ȸʯΪԭ���Ʊ�CuSO4��5H2O���������ͼ��
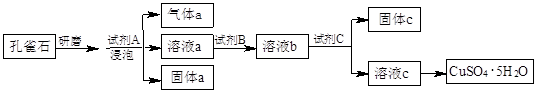
��1����ĥ��ȸʯ��Ŀ���� �����ݿ�ȸʯ���Լ�A��ѡ�ù�����ϡ���ᣬ�����a�� (�ѧʽ)��
��2���Լ�B��Ŀ���ǽ���Һ�е�Fe2+ת��ΪFe3+�����Լ�B��ѡ�� ������ţ���
A������KMnO4��Һ B��˫��ˮ C��Ũ���� D����ˮ
��Ӧ�����ӷ���ʽΪ�� ��
��3���Լ�C���ڵ�����ҺpH��ʹFe3+ת��Ϊ�������Է��롣���Լ�C��ѡ�� ������ţ���
A��ϡ���� B��NaOH��Һ C����ˮ D��CuO
����C�Ļ�ѧʽΪ ��
��4��1 mol����ͨ�����ȵ�Cu2(OH)2 CO3���Բ���1.5 mol����ͭ����÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��������Fe(OH)3��Ksp=1��10-39����Ҫ����Һ�е�Fe3+ת��ΪFe(OH)3������ʹ��Һ��c(Fe3+)������1��10��3 mol��L�����轫��Һ������pH= ��
��1������4�֣��������������1�֣���������Ӧ���ʣ�1�֣�[������˼����]
SiO2��2�֣�
��2������4�֣�B��2�֣� 2Fe2++2H++H2O2=2Fe3++2H2O��2�֣���δ��ƽ1�֣�дΪ��ѧ����ʽ2FeSO4 + H2SO4 + H2O2 = Fe2(SO4)3 + 2H2O����ȷ1�֣�
��3������4�֣�D��2�֣� Fe(OH)3��2�֣�
��4������3�֣�3Cu2(OH)2CO3 + 4NH3 6Cu + 3CO2 + 9H2O +2 N2����ѧʽ1�֡���ƽ��1�֣�����1�֡���
6Cu + 3CO2 + 9H2O +2 N2����ѧʽ1�֡���ƽ��1�֣�����1�֡���
��5������2�֣�2
SiO2��2�֣�
��2������4�֣�B��2�֣� 2Fe2++2H++H2O2=2Fe3++2H2O��2�֣���δ��ƽ1�֣�дΪ��ѧ����ʽ2FeSO4 + H2SO4 + H2O2 = Fe2(SO4)3 + 2H2O����ȷ1�֣�
��3������4�֣�D��2�֣� Fe(OH)3��2�֣�
��4������3�֣�3Cu2(OH)2CO3 + 4NH3
 6Cu + 3CO2 + 9H2O +2 N2����ѧʽ1�֡���ƽ��1�֣�����1�֡���
6Cu + 3CO2 + 9H2O +2 N2����ѧʽ1�֡���ƽ��1�֣�����1�֡�����5������2�֣�2
�����������1����ĥ��ȸʯ��Ŀ����Ϊ���������ʸ��죬���Դ�Ϊ���������Ӵ�������ӿ췴Ӧ���ʡ������ܹ����в��ܵĹ���Ϊ����SiO2��
��2����Fe2+ת��ΪFe3+ Ӧ�ü������������Ҳ��ܴ����������ӣ�������˫��ˮ��ѡB����Ӧ�����ӷ���ʽΪ��2Fe2++2H++H2O2=2Fe3++2H2O��
��3����ΪĿ���Ʒ��CuSO4��5H2O���壬���Ե���pHֵʹ�����ӳ���Ӧѡ�õ��Լ����ܴ����������ӣ�����ѡ�ù���CuO�����Գ�����cΪFe(OH)3 ������CuO��
��4����ʽ̼��ͭ��ͨ�백������ͭ�������ɣ�˵��ͭ����ԭ��NH3����ԭ������Ϊ1mol������ԭ�õ�1.5molͭ���ʣ�����ͭ+2��0�ۣ���֪ת�Ƶ���3mol����ʧ�����غ㣬����NH3ʧȥ����ҲΪ3mol�����Կ��Ʋ��NH3������Ϊ��N2�����Կ��Ե�֪�������ﻹ��CO2��H2O�������г���Ӧ��NH3+ Cu2(OH)2 CO3��Cu+ N2+CO2+H2O��Ȼ�����������ԭ��Ӧ����ʽ��ƽ�ã�3Cu2(OH)2CO3 + 4NH3
 6Cu + 3CO2 + 9H2O +2 N2 ��
6Cu + 3CO2 + 9H2O +2 N2 ����5������Fe(OH)3�ܽ�ƽ��Fe(OH)3 (s)
 Fe3+ (aq)+3 OH- (aq)�ɵ�Ksp ="c(" Fe3+ ) ��c3(OH- )= 1��10-39 ������c(Fe3+)=1��10��3 mol��L���빫ʽ�У������c(OH- )= 1��10-12 ������c(H+ )="Kw/" c(OH- )= 10-2 ������pH=2��
Fe3+ (aq)+3 OH- (aq)�ɵ�Ksp ="c(" Fe3+ ) ��c3(OH- )= 1��10-39 ������c(Fe3+)=1��10��3 mol��L���빫ʽ�У������c(OH- )= 1��10-12 ������c(H+ )="Kw/" c(OH- )= 10-2 ������pH=2��
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ



 (a+b) mol
(a+b) mol

