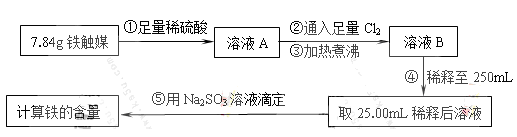
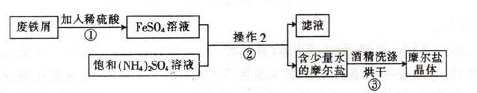
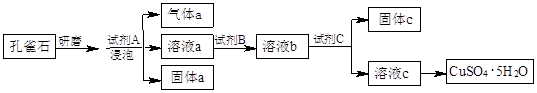��Ŀ����
ͭ�����ֳ�����������CuO��Cu2O��ijѧϰС��ȡ0.98 g(�þ�����ƽ����)Cu(OH)2���壬���������ͭ�����������ɣ����������¶ȱ仯��������ͼ1��ʾ��
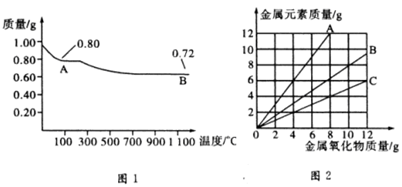
���⣬ijͬѧ������������ʾ����������������������Ԫ�������Ĺ�ϵ���ߣ���ͼ2��ʾ�������з�����ȷ����
A��ͼ1�в���A��B�Ļ�ѧʽ�ֱ�ΪCu2O��CuO
B��ͼ1���������й�����0.26 g H2O
C��ͼ2���������У���ʾCuO����������CuԪ�������Ĺ�ϵ����������A
D��ͼ2�л��ƴ�������߹�2��

���⣬ijͬѧ������������ʾ����������������������Ԫ�������Ĺ�ϵ���ߣ���ͼ2��ʾ�������з�����ȷ����
A��ͼ1�в���A��B�Ļ�ѧʽ�ֱ�ΪCu2O��CuO
B��ͼ1���������й�����0.26 g H2O
C��ͼ2���������У���ʾCuO����������CuԪ�������Ĺ�ϵ����������A
D��ͼ2�л��ƴ�������߹�2��
D
���������A����0.98 g Cu(OH)2��֪�����ʵ���Ϊ0.01 mol����ȫ������CuO��������Ϊ0.01 mol��80 g?mol-1��0.8g������A����CuO����ȫ������Cu2O��������Ϊ0.005 mol��144 g?mol-1��0.72g������B����Cu2O����A����ȷ��B�����ݻ�ѧ����ʽ4Cu2O
 4Cu+2O2��֪����Ӧ������������������0.26 g��ˮ�������������ͣ���B����C��CuO����������CuԪ�ص�������ϵ����CuO��Cu2O��������Ϊ10g���㣩Ϊ��
4Cu+2O2��֪����Ӧ������������������0.26 g��ˮ�������������ͣ���B����C��CuO����������CuԪ�ص�������ϵ����CuO��Cu2O��������Ϊ10g���㣩Ϊ��CuO��Cu Cu2O��2Cu
80 64 144 128
10g 8g 10g 8.89g
�۲�ͼ2��֪��B��������ͭ������������Ԫ�������Ĺ�ϵ���ߣ���ʾ����CuO����A���ϵ��κ�һ�㶼��ʾ���������������С��������������Ԫ�ص���������C����D��ͼ2��A�����ϵ��κ�һ�㶼��ʾ���������������С��������������Ԫ�ص�����������A���ߴ�����Cѡ���֪B������ȷ��C�����ϵ��κ�һ�㶼��ʾ�������������������������������Ԫ�ص��������Ҷ��ߵ�����֮����2:1,������������ΪCuO2������C����Ҳ����ģ���D��ȷ����ѡD��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ