��Ŀ����
�蹸���ɷ�ֹ��ҵ��ˮ��������������Ṹ���������з�Ӧ·�߿ɵõ�E��R�����蹸�������ַ�Ӧ������ȥ��
��1���蹸��E���Ʊ�

��A����������Ҫ��Ӫ������______ˮ���Ƶã�����ࡱ������֬�������ʡ�����
��B�����Ƶ�Cu��OH��2��Ӧ����D���仯ѧ����ʽΪ______��
��D���Ӿ۷�Ӧ����E��E�Ľṹ��ʽΪ______��
��2���蹸��R���Ʊ�

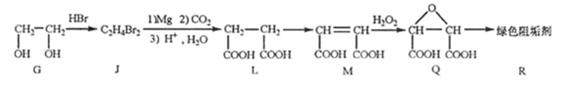
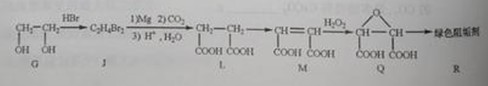
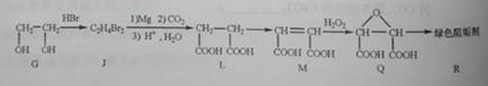
��G��JΪȡ����Ӧ��J�Ľṹ��ʽΪ______��
��Jת��ΪL�Ĺ����У�L���������ӵ�̼ԭ����Դ��______��
����L�Ʊ�M�ķ�ӳ��������Ϊ��
HOOCCH2CH2COOH+HB��
HOOCCH2CHBrCOOH+HBr��______��______���û�ѧ����ʽ��ʾ��
��1molQ��ͬ���칹��T��̼����֧����������NaHCO3��Һ���ò���2molCO2��T�Ľṹ��ʽΪ______��ֻдһ�֣���
��1���蹸��E���Ʊ�

��A����������Ҫ��Ӫ������______ˮ���Ƶã�����ࡱ������֬�������ʡ�����
��B�����Ƶ�Cu��OH��2��Ӧ����D���仯ѧ����ʽΪ______��
��D���Ӿ۷�Ӧ����E��E�Ľṹ��ʽΪ______��
��2���蹸��R���Ʊ�

��G��JΪȡ����Ӧ��J�Ľṹ��ʽΪ______��
��Jת��ΪL�Ĺ����У�L���������ӵ�̼ԭ����Դ��______��
����L�Ʊ�M�ķ�ӳ��������Ϊ��
HOOCCH2CH2COOH+HB��
| ���� |
| �� |
��1molQ��ͬ���칹��T��̼����֧����������NaHCO3��Һ���ò���2molCO2��T�Ľṹ��ʽΪ______��ֻдһ�֣���
��1������֬ˮ�����ɸ�֬������ͣ��ʴ�Ϊ����֬��
����B��DΪȩ�����Ƶ�Cu��OH��2�������������ᣬ��B�ķ���ʽ��֪�������к���1��C=C˫����1��-CHO����A�м�̼ԭ���ϵ��ǻ�������ȥ��Ӧ��Ȼ���컯Ϊȩ������BΪCH2=CHCHO��DΪCH2=CHCOOH��B�����Ƶ�Cu��OH��2��Ӧ����D�Ļ�ѧ����ʽΪCH2=CHCHO+2Cu��OH��2
CH2=CHCOOH+Cu2O+2H2O��
�ʴ�Ϊ��CH2=CHCHO+2Cu��OH��2
CH2=CHCOOH+Cu2O+2H2O��
��B��DΪȩ�����Ƶ�Cu��OH��2��������������D��DΪCH2=CHCOOH������˫���������Ӿ۷�Ӧ�ɵ�E����E�Ľṹ��ʽΪ

���ʴ�Ϊ��

��
��2����G��JΪȡ����Ӧ����G��J�ķ���ʽ��֪��G��2��-OH��Brԭ��ȡ��������BrCH2CH2Br���ʴ�Ϊ��BrCH2CH2Br��
�ڴ���Ŀ��Ϣ�пɵó���J��L������ֻ��CO2���ṩ̼ԭ�ӣ�����L���������ӵ�̼ԭ����Դ��CO2���ʴ�Ϊ��CO2��
��HOOCCH2CHBrCOOH�ڼ��������·�����ȥ��Ӧ������NaOOCCH=CHCOONa���ټ����ữ��ʹNaOOCCH=CHCOONaת��ΪHOOCCH=CHCOOH���ʷ�Ӧ����ʽΪ��HOOCCH2CHBrCOOH+3NaOH
NaOOCCH=CHCOONa+NaBr+3H2O��NaOOCCH=CHCOONa+H2SO4��HCOOCH=CHCOOH+Na2SO4��
�ʴ�Ϊ��HOOCCH2CHBrCOOH+3NaOH
NaOOCCH=CHCOONa+NaBr+3H2O��NaOOCCH=CHCOONa+H2SO4��HCOOCH=CHCOOH+Na2SO4��
��1 molT�ܺ�NaHCO3���ò���2 molCO2����֪T�бغ������Ȼ���̼����֧��������������T�Ľṹ��

��

���ʴ�Ϊ��

�ȣ�
����B��DΪȩ�����Ƶ�Cu��OH��2�������������ᣬ��B�ķ���ʽ��֪�������к���1��C=C˫����1��-CHO����A�м�̼ԭ���ϵ��ǻ�������ȥ��Ӧ��Ȼ���컯Ϊȩ������BΪCH2=CHCHO��DΪCH2=CHCOOH��B�����Ƶ�Cu��OH��2��Ӧ����D�Ļ�ѧ����ʽΪCH2=CHCHO+2Cu��OH��2
| �� |
�ʴ�Ϊ��CH2=CHCHO+2Cu��OH��2
| �� |
��B��DΪȩ�����Ƶ�Cu��OH��2��������������D��DΪCH2=CHCOOH������˫���������Ӿ۷�Ӧ�ɵ�E����E�Ľṹ��ʽΪ

���ʴ�Ϊ��

��
��2����G��JΪȡ����Ӧ����G��J�ķ���ʽ��֪��G��2��-OH��Brԭ��ȡ��������BrCH2CH2Br���ʴ�Ϊ��BrCH2CH2Br��
�ڴ���Ŀ��Ϣ�пɵó���J��L������ֻ��CO2���ṩ̼ԭ�ӣ�����L���������ӵ�̼ԭ����Դ��CO2���ʴ�Ϊ��CO2��
��HOOCCH2CHBrCOOH�ڼ��������·�����ȥ��Ӧ������NaOOCCH=CHCOONa���ټ����ữ��ʹNaOOCCH=CHCOONaת��ΪHOOCCH=CHCOOH���ʷ�Ӧ����ʽΪ��HOOCCH2CHBrCOOH+3NaOH
| �� |
| �� |
�ʴ�Ϊ��HOOCCH2CHBrCOOH+3NaOH
| �� |
| �� |
��1 molT�ܺ�NaHCO3���ò���2 molCO2����֪T�бغ������Ȼ���̼����֧��������������T�Ľṹ��

��

���ʴ�Ϊ��

�ȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
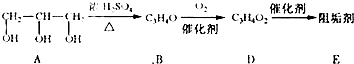

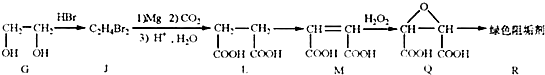
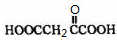 ��
��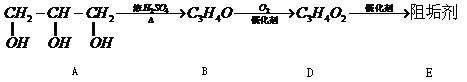
 ��T�Ľṹ��ʽΪ ��ֻдһ�֣���
��T�Ľṹ��ʽΪ ��ֻдһ�֣���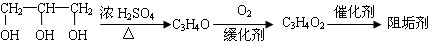
 ____��
____��
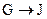 Ϊȡ����Ӧ��J�Ľṹ��ʽΪ__________________��
Ϊȡ����Ӧ��J�Ľṹ��ʽΪ__________________��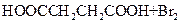

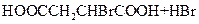 ��_____��_____���û�ѧ����ʽ��ʾ����
��_____��_____���û�ѧ����ʽ��ʾ����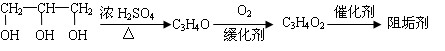
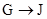 Ϊȡ����Ӧ��J�Ľṹ��ʽΪ__________________��
Ϊȡ����Ӧ��J�Ľṹ��ʽΪ__________________��
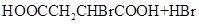 ��_____��_____���û�ѧ����ʽ��ʾ����
��_____��_____���û�ѧ����ʽ��ʾ����