��Ŀ����
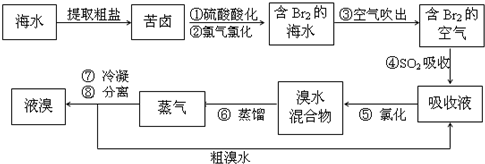
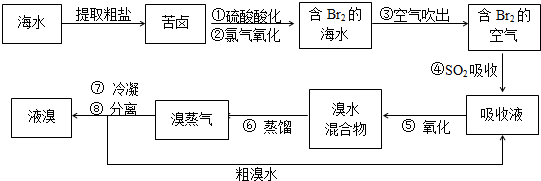
�������������գ���Ŀǰ����ˮ���塱������Ҫ����֮һ���Ժ�ˮ���κ�õ��Ŀ�±Ϊԭ�ϣ�����Ĺ����������£�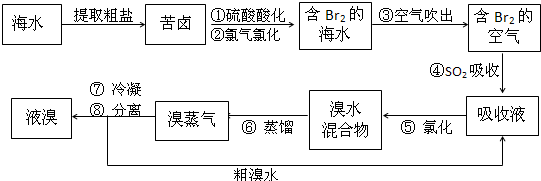
�ش��������⣺
��1������ڵ����ӷ���ʽΪ
��2������������У�������¶�Ҫ������80-900C���¶ȹ�����Ͷ�������������������
��3������ں��Ѿ��õ��˺���ĺ�ˮ��Ϊʲô����ֱ�ӽ�������õ�Һ�壬��Ҫ����һϵ�к���������ԭ����

�ش��������⣺
��1������ڵ����ӷ���ʽΪ
Cl2+2Br-�TBr2+2Cl-
Cl2+2Br-�TBr2+2Cl-
������ܵ����ӷ���ʽΪBr2+SO2+2H2O�T4H++SO42-+2Br-
Br2+SO2+2H2O�T4H++SO42-+2Br-
����2������������У�������¶�Ҫ������80-900C���¶ȹ�����Ͷ�������������������
�������¶ȹ�����������ˮ�����Ͳ������������
�������¶ȹ�����������ˮ�����Ͳ������������
����3������ں��Ѿ��õ��˺���ĺ�ˮ��Ϊʲô����ֱ�ӽ�������õ�Һ�壬��Ҫ����һϵ�к���������ԭ����
ԭ�������Ũ��̫�͡�����Ч�ʵͳɱ�̫��
ԭ�������Ũ��̫�͡�����Ч�ʵͳɱ�̫��
����������1��Cl2�ɽ�Br-����ΪBr2��Br2��SO2����������ԭ��Ӧ����HBr��H2SO4���Դ���д���ӷ���ʽ��
��2��������80-90�棬��ֹ������Br2��ˮ�����ӷ���
��3���Ȼ���ĺ�ˮ��Ȼ�����嵥�ʣ���Ũ�ȵͣ������ͣ�
��2��������80-90�棬��ֹ������Br2��ˮ�����ӷ���
��3���Ȼ���ĺ�ˮ��Ȼ�����嵥�ʣ���Ũ�ȵͣ������ͣ�
����⣺��1��Cl2�ɽ�Br-����ΪBr2��Br2��SO2����������ԭ��Ӧ����HBr��H2SO4����Ӧ�����ӷ���ʽΪBr2+SO2+2H2O=4H++2Br-+SO42-��
�ʴ�Ϊ��Cl2+2Br-�TBr2+2Cl-��Br2+SO2+2H2O�T4H++SO42-+2Br-��
��2���������������У�������¶�Ϊ��Ҫ������80-90�棬ӦΪ�¶ȹ��ߣ�����ˮ�����ų���������ˮ���ӣ� �¶ȹ��ͣ��岻����ȫ�����������ʵͣ��¶ȹ�����Ͷ��������������ʴ�Ϊ���������¶ȹ�����������ˮ�����Ͳ��������������
��3���Ȼ���ĺ�ˮ��Ȼ�����嵥�ʣ���Ũ�ȵͣ������ͣ�Ӧ����������������SO2���ա��Ȼ����Ĺ��̽��и������ʴ�Ϊ��ԭ�������Ũ��̫�͡�����Ч�ʵͳɱ�̫�ߣ�
�ʴ�Ϊ��Cl2+2Br-�TBr2+2Cl-��Br2+SO2+2H2O�T4H++SO42-+2Br-��
��2���������������У�������¶�Ϊ��Ҫ������80-90�棬ӦΪ�¶ȹ��ߣ�����ˮ�����ų���������ˮ���ӣ� �¶ȹ��ͣ��岻����ȫ�����������ʵͣ��¶ȹ�����Ͷ��������������ʴ�Ϊ���������¶ȹ�����������ˮ�����Ͳ��������������
��3���Ȼ���ĺ�ˮ��Ȼ�����嵥�ʣ���Ũ�ȵͣ������ͣ�Ӧ����������������SO2���ա��Ȼ����Ĺ��̽��и������ʴ�Ϊ��ԭ�������Ũ��̫�͡�����Ч�ʵͳɱ�̫�ߣ�
������������Ҫ�Ժ�ˮ��ѧ��Դ������Ϊ�����������֪ʶ��϶࣬������ѧ������֪ʶ��������ͽ�������������

��ϰ��ϵ�д�
 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д� ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�
�����Ŀ
�������������գ���Ŀǰ����ˮ���塱������Ҫ����֮һ���乤���������£����в���ȷ���ǣ�������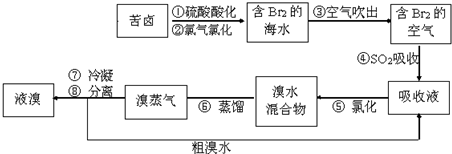

| A����������������ữ�ɷ�ֹCl2��Br2��ˮ��Ӧ | B������ܵ����ӷ���ʽBr2+SO2+H2O=2H++2Br-+SO42- | C���������ڱ���λ��4����VIIA�� | D���������������������õ�Һ������ˮ�Ļ������÷�Һ©�� |