��Ŀ����
(1)Ti��ԭ������Ϊ22��Tiλ��Ԫ�����ڱ��е�_______ ���ڣ���____�塣
(2)����ټ�Fe��Ŀ����_________�� �������ȴ��Ŀ����______________________��

(4) 800��ʱ����ӦTiCl4+2Mg = 2MgCl2+Ti��Ar�����н��е�������_________________________��
(2)��Fe3+��ԭΪFe2+ ������������롢��õ���FeSO4��7H2O
(3) FeSO4��7H2O��ʯ�ң���̼��ơ��ϼ
(4)��ֹ������Mg(Ti)������е�O2����CO2��N2������

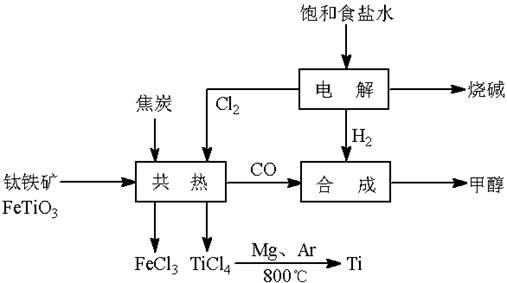
(13��) ��(Ti)����Ϊ��������֮��ĵ�������������ͼ��ʾ�����ѳ����ȼ�ͼ״�����ɲ�ҵ�����Դ�������Դ�����ʣ����ٻ�����Ⱦ������д���пհף�
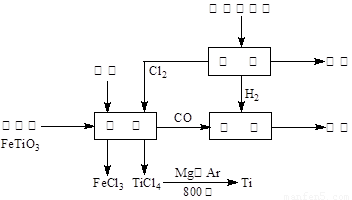
��1����ⱥ��ʳ��ˮʱ�������ĵ缫��ӦΪ ��
��2��д���������뽹̿��Cl2�����Ƶ����Ȼ��ѵĻ�ѧ����ʽ________________________��
��3����֪����Mg(s) + Cl2(g)��MgCl2(s)����H = �C 641 kJ/mol
��Ti(s) + 2Cl2(g)��TiCl4(s)����H = �C770 kJ/mol
��2Mg(s) + TiCl4(s)��2MgCl2(s) + Ti(s)����H�� ��
��Ӧ2Mg(s) + TiCl4(s) 2MgCl2(s) + Ti(s)����Ar�����н��е�������
��
2MgCl2(s) + Ti(s)����Ar������������
��
��4����������ҵ���У��ϳ�96 t �״�����������H2 t (�������������������ʵ��κ���ʧ)��
��5���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء���֪��ȼ�ϵ�ص��ܷ�ӦʽΪ��2CH3OH + 3O2 + 4OH����2CO32�� + 6H2O����ȼ�ϵ�ط�����Ӧʱ��������Һ��pH (���������С�����䡱)���õ���и����ϵĵ缫��Ӧ��________________________________________________��


 2MgCl4��Ti��Ar������������____________________��
2MgCl4��Ti��Ar������������____________________�� CH3OCH3(g)+H2O(g)����H=" -23.5" kJ/mol
CH3OCH3(g)+H2O(g)����H=" -23.5" kJ/mol


 2MgCl2(s)
+ Ti����Ar�����н��е������ǣ�
______________________________________
��
2MgCl2(s)
+ Ti����Ar�����н��е������ǣ�
______________________________________
�� ��4���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء��õ���и����ϵĵ缫��Ӧʽ��
��
��4���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء��õ���и����ϵĵ缫��Ӧʽ��
��