��Ŀ����
��ľ���и������Σ���Ҫ�ɷ���̼��ء��ִӲ�ľ������ȡ���Σ�����ʵ��������е�SO42-��CO32-��
Cl-��
��1���Ӳ�ľ������ȡ���ε�ʵ�����˳�����£�
�ٳ�����Ʒ
���ܽ�ͳ���
��______________________
��______________________
����ȴ�ᾧ��
��2�� ��������ƽ(ָ�����ϵ�)������Ʒʱ����ָ��ƫ���ұߣ����ʾ��__________
A.�����أ���Ʒ��
B.�����ᣬ������
C.�����أ�������
D.�������أ���Ʒ��
��3���ڽ��Тڢۢܲ���ʱ��Ҫ�õ��������������÷ֱ���__________ ��___________ ��___________��
��4�����Ƶõ�������������Թܣ�������ˮ�ܽⲢ����Һ�ֳ����ݣ���װ����֧�Թ��
���ڵ�һ֧�Թ������ϡ���ᣬ�ɹ۲���________ ���ɣ�֤����Һ����_______ ���ӡ�
���ڵڶ�֧�Թ����������ϡ������ټ����Ȼ�����Һ���ɹ۲���________���ɣ�֤����Һ����___________���ӡ�
���ڵ���֧�Թ����������ϡ������ټ�����������Һ���ɹ۲���________���ɣ�֤����Һ����___________���ӡ�
Cl-��
��1���Ӳ�ľ������ȡ���ε�ʵ�����˳�����£�
�ٳ�����Ʒ
���ܽ�ͳ���
��______________________
��______________________
����ȴ�ᾧ��
��2�� ��������ƽ(ָ�����ϵ�)������Ʒʱ����ָ��ƫ���ұߣ����ʾ��__________
A.�����أ���Ʒ��
B.�����ᣬ������
C.�����أ�������
D.�������أ���Ʒ��
��3���ڽ��Тڢۢܲ���ʱ��Ҫ�õ��������������÷ֱ���__________ ��___________ ��___________��
��4�����Ƶõ�������������Թܣ�������ˮ�ܽⲢ����Һ�ֳ����ݣ���װ����֧�Թ��
���ڵ�һ֧�Թ������ϡ���ᣬ�ɹ۲���________ ���ɣ�֤����Һ����_______ ���ӡ�
���ڵڶ�֧�Թ����������ϡ������ټ����Ȼ�����Һ���ɹ۲���________���ɣ�֤����Һ����___________���ӡ�
���ڵ���֧�Թ����������ϡ������ټ�����������Һ���ɹ۲���________���ɣ�֤����Һ����___________���ӡ�
��1�����ˣ�����
��2��B
��3�����裻����������
��4�����壻CO32- ����ɫ������SO42- ����ɫ������Cl-
��2��B
��3�����裻����������
��4�����壻CO32- ����ɫ������SO42- ����ɫ������Cl-

��ϰ��ϵ�д�
�����Ŀ
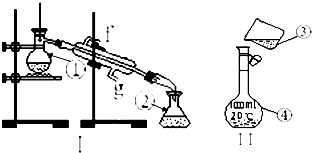 ���������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�
���������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�