��Ŀ����
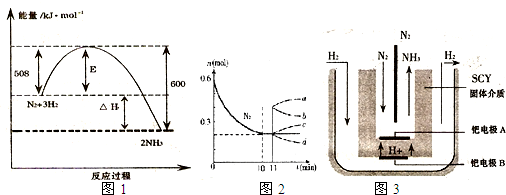
���ŶԺϳɰ��о��ķ�չ��2001����λϣ����ѧ������˵��ϳɰ��ķ��������ڳ�ѹ�°��������ú���ϡ�͵ĵ������ֱ�ͨ��һ�����ȵ�570��ĵ����У����ø����ӵ����Ե�SCY�մɣ��ܴ���H+��Ϊ���ʣ�������������������ϵĽ����ٶྦྷ��Ĥ���缫��ʵ���˳�ѹ��570�������¸�ת���ʵĵ�ⷨ�ϳɰ���װ����ͼ���������й�˵���в���ȷ����

[ ]
A����ⷨ�ϳɰ��ĵ���������ˮ���������Һ���ܼ�
B���ٵ缫A�ĵ缫��ӦʽΪ��N2+6e-+6H+==2NH3
C���ٵ缫B���ӵ��ǵ�Դ������
D������0.3mol����ת��ʱ����0.12mol NH3����
B���ٵ缫A�ĵ缫��ӦʽΪ��N2+6e-+6H+==2NH3
C���ٵ缫B���ӵ��ǵ�Դ������
D������0.3mol����ת��ʱ����0.12mol NH3����
A

��ϰ��ϵ�д�
 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
�����Ŀ


 2NH3��g������H=
-92��4 kJ��mol-1����ش�
2NH3��g������H=
-92��4 kJ��mol-1����ش� ����N2��ת���ʧ�1=�ߣߣߣߣߣ���ʱ����Ӧ���ȣߣߣߣߣ�kJ�����¶��ºϳɰ���Ӧ��ƽ�ⳣ��K=�ߣߣߣߣߣ�ֻ���г����ֱ���ʽ����
����N2��ת���ʧ�1=�ߣߣߣߣߣ���ʱ����Ӧ���ȣߣߣߣߣ�kJ�����¶��ºϳɰ���Ӧ��ƽ�ⳣ��K=�ߣߣߣߣߣ�ֻ���г����ֱ���ʽ����