��Ŀ����
��֪AΪ��ѧ��ѧ�е�һ������ɫ�Σ�B��CΪ�ճ������г����Ľ������ʣ�D��GΪ��ɫ��ζ���壬������֮���ת����ϵ����ͼ�������ö��Ե缫�����ַ�Ӧ��������ȥ����

��ش��������⣺
�ŵ��A��Һ��������ӦʽΪ_____________��
��A��Һ�е�A��Na2O2�����ʵ���֮��Ϊ1:1��Ӧʱ���ܻ�ѧ����ʽΪ____________��
��E��ϡ��Һ��F��Һ��Ӧ�����ӷ���ʽΪ____________��
�ȵ��100 mL��A����Һһ��ʱ��Ͽ���·��ȡ���缫����õ�����Һ��pHΪ1��������Һ������䣩�������õ�������D�ڱ�״���µ����Ϊ_____________ mL��
����H��ҺΪֻ����һ���ʵ���Һ������Ũ��Ϊ1mol/L���ֱ����ķ�200mL��H����Һ�м���B ��C�ĺϽ𣬵õ����±���ʾ�����ݣ��Իش��й����⡣
�ŵ��A��Һ��������ӦʽΪ_____________��
��A��Һ�е�A��Na2O2�����ʵ���֮��Ϊ1:1��Ӧʱ���ܻ�ѧ����ʽΪ____________��
��E��ϡ��Һ��F��Һ��Ӧ�����ӷ���ʽΪ____________��
�ȵ��100 mL��A����Һһ��ʱ��Ͽ���·��ȡ���缫����õ�����Һ��pHΪ1��������Һ������䣩�������õ�������D�ڱ�״���µ����Ϊ_____________ mL��
����H��ҺΪֻ����һ���ʵ���Һ������Ũ��Ϊ1mol/L���ֱ����ķ�200mL��H����Һ�м���B ��C�ĺϽ𣬵õ����±���ʾ�����ݣ��Իش��й����⡣
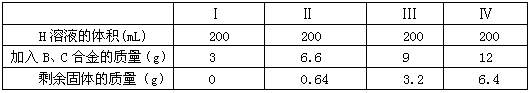
�ٸ��ݱ��е����ݿ�����Ͻ���B ��C �����ʵ�����֮��Ϊ ��
�ڸ��ݱ������ݷ�����Ӧ�����Һ�д��ڵĽ����������� �������ӷ��ű�ʾ����
�ڸ��ݱ������ݷ�����Ӧ�����Һ�д��ڵĽ����������� �������ӷ��ű�ʾ����
��1��4OH--4e-=2H2O+O2������2H2O-4e-=O2��+4H+����
��2��2Na2O2��2Cu(NO3)2��2H2O=2Cu(OH)2����4NaNO3��O2����
��3��3Fe2+��NO3-��4H+==3Fe3+��NO����2H2O��
��4��56��
��5����7:8
��Fe3+��Fe2+��Cu2+
��2��2Na2O2��2Cu(NO3)2��2H2O=2Cu(OH)2����4NaNO3��O2����
��3��3Fe2+��NO3-��4H+==3Fe3+��NO����2H2O��
��4��56��
��5����7:8
��Fe3+��Fe2+��Cu2+

��ϰ��ϵ�д�
 �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ
 ��2012?����һģ����֪AΪ��ѧ��ѧ�е�һ���Σ�B��CΪ�ճ������г����Ľ�����ͨ��������D��GΪ��ɫ��ζ���壮��֪�ö��Ե缫���A��Һһ��ʱ�����ֻ��C��D��E��ϡ��Һ��������֮���ת����ϵ��ͼ�����ַ�Ӧ��������ȥ����
��2012?����һģ����֪AΪ��ѧ��ѧ�е�һ���Σ�B��CΪ�ճ������г����Ľ�����ͨ��������D��GΪ��ɫ��ζ���壮��֪�ö��Ե缫���A��Һһ��ʱ�����ֻ��C��D��E��ϡ��Һ��������֮���ת����ϵ��ͼ�����ַ�Ӧ��������ȥ����

