��Ŀ����
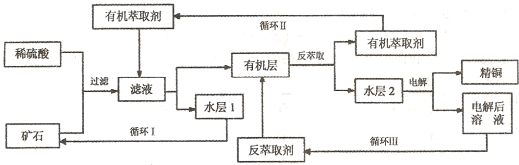
ijͭ��ʯ������ͭ��������ͭ�������������ʹ�����ʯ��SiO2��,�ֲ���������ӿ�ʯ����ȡͭ��������ͼ����

��֪��
�ٵ���ʯ����������������̫��ʱ������������������Ļ��Һ����ͭ��
�ڷ���ȡ���ˮ��������ͭ��Һ��Cu2+Ũ��ԼΪ50g/L��
�ش��������⣺
��1����ʯ��ϡ�������������������ͭ�����ķ�ӦΪ��Cu2O+2H+==Cu2++Cu+H2O����д���ù����з�������һ��������ԭ��Ӧ�����ӷ���ʽ��______________________��
��2��д���ö��Ե缫���ˮ��ĵ���ܷ�Ӧ����ʽ��_________________________��
��3��ѭ���з���ȡ��B����Ҫ�ɷ���___________________��
��4��ijͭ��ʯ��Ʒ�У�������������ͭ����������������ʯ�������ʡ�ȡ�ÿ�ʯ��Ʒ200.0g����100mL1.0mol/LH2SO4��Һ��ȡ�������10mL 1.0mol/L Fe2(SO4)3��Һ����ʹͭȫ����������ȡҺ����ֵ���ɵõ� 6.4gCu����ͭ��ʯ��Ʒ��������ͭ��������������������
�ٵ���ʯ����������������̫��ʱ������������������Ļ��Һ����ͭ��
�ڷ���ȡ���ˮ��������ͭ��Һ��Cu2+Ũ��ԼΪ50g/L��
�ش��������⣺
��1����ʯ��ϡ�������������������ͭ�����ķ�ӦΪ��Cu2O+2H+==Cu2++Cu+H2O����д���ù����з�������һ��������ԭ��Ӧ�����ӷ���ʽ��______________________��
��2��д���ö��Ե缫���ˮ��ĵ���ܷ�Ӧ����ʽ��_________________________��
��3��ѭ���з���ȡ��B����Ҫ�ɷ���___________________��
��4��ijͭ��ʯ��Ʒ�У�������������ͭ����������������ʯ�������ʡ�ȡ�ÿ�ʯ��Ʒ200.0g����100mL1.0mol/LH2SO4��Һ��ȡ�������10mL 1.0mol/L Fe2(SO4)3��Һ����ʹͭȫ����������ȡҺ����ֵ���ɵõ� 6.4gCu����ͭ��ʯ��Ʒ��������ͭ��������������������
��1��Cu+2Fe3+==2Fe2++ Cu2+
��2��2CuSO4 + 2H2O O2��+ 2Cu + 2H2SO4
O2��+ 2Cu + 2H2SO4
��3��H2SO4
��4��Cu2O��3.6% ��Fe2O3��3.2%
��2��2CuSO4 + 2H2O
 O2��+ 2Cu + 2H2SO4
O2��+ 2Cu + 2H2SO4��3��H2SO4
��4��Cu2O��3.6% ��Fe2O3��3.2%

��ϰ��ϵ�д�
 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
�����Ŀ
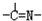 ���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=
���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=


 ���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=
���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=
 ���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=
���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=