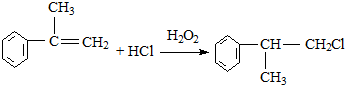��Ŀ����
�ش��������⣺
��1����Al2O3��Ni������̬���ᷢ�����з�Ӧ��
���ᣨg��=CO ��g��+H2O ��g����H1=+34.0kJ/mol
���ᣨg��=CO2 ��g��+H2��g����H2=-7.0kJ/mol
�ڸ������£���̬CO2 ����̬H2 ������̬CO����̬H2O���Ȼ�ѧ����ʽΪ
��2������ƽ�����ʢ��ǿ��ԭ��Һ̬�£�N2H4����ǿ������Һ̬˫��ˮ��H2O2��������0.4molҺ̬�º�0.8mol Һ̬H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ���������൱��25�桢101kPa�²�õ�����������Ӧ���Ȼ�ѧ����ʽΪ
��3����֪��C��s��+O2��g��=CO2��g����H=-393.5kJ?mol-1
2H2��g��+O2��g��=2H2O��g����H=-483.6kJ?mol-1
����0.2mol��̿�ۺ�������ɵ����������һ��������������ȫȼ�գ����ų�63.53kJ��������������C��H2�����ʵ���֮��Ϊ
��1����Al2O3��Ni������̬���ᷢ�����з�Ӧ��
���ᣨg��=CO ��g��+H2O ��g����H1=+34.0kJ/mol
���ᣨg��=CO2 ��g��+H2��g����H2=-7.0kJ/mol
�ڸ������£���̬CO2 ����̬H2 ������̬CO����̬H2O���Ȼ�ѧ����ʽΪ
CO2��g��+H2��g��=CO��g��+H2O��g����H=+41.0kJ/mol
CO2��g��+H2��g��=CO��g��+H2O��g����H=+41.0kJ/mol
����2������ƽ�����ʢ��ǿ��ԭ��Һ̬�£�N2H4����ǿ������Һ̬˫��ˮ��H2O2��������0.4molҺ̬�º�0.8mol Һ̬H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ���������൱��25�桢101kPa�²�õ�����������Ӧ���Ȼ�ѧ����ʽΪ
N2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-641.75kJ?mol-1
N2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-641.75kJ?mol-1
����3����֪��C��s��+O2��g��=CO2��g����H=-393.5kJ?mol-1
2H2��g��+O2��g��=2H2O��g����H=-483.6kJ?mol-1
����0.2mol��̿�ۺ�������ɵ����������һ��������������ȫȼ�գ����ų�63.53kJ��������������C��H2�����ʵ���֮��Ϊ
1��1
1��1
����������1�����ݸ�˹���������㻯ѧ��Ӧ���ʱ䣬�����Ȼ�ѧ����ʽ����д��������д���ɣ�
��2�������Ȼ�ѧ����ʽ���ʱ�ͷ�Ӧ����ʽϵ��֮��Ĺ�ϵ�Լ��Ȼ�ѧ����ʽ����д�������ش�
��3���������ʵ����뷴Ӧ�ų������������ȣ������Ȼ�ѧ��Ӧ����ʽ����C��H2ȼ�շų����������Դ������㣮
��2�������Ȼ�ѧ����ʽ���ʱ�ͷ�Ӧ����ʽϵ��֮��Ĺ�ϵ�Լ��Ȼ�ѧ����ʽ����д�������ش�
��3���������ʵ����뷴Ӧ�ų������������ȣ������Ȼ�ѧ��Ӧ����ʽ����C��H2ȼ�շų����������Դ������㣮
����⣺��1����֪���ټ��ᣨg��=CO ��g��+H2O ��g����H1=+34.0kJ/mol��
�ڼ��ᣨg��=CO2 ��g��+H2��g����H2=-7.0kJ/mol��
��̬CO2����̬H2 ������̬CO����̬H2O�Ļ�ѧ����ʽΪCO2��g��+H2��g��=CO��g��+H2O��g�����Ը��ݢ�-�ڵõ���
���ԡ�H=34.0kJ/mol-��-7.0kJ/mol��=+41.0kJ/mol����CO2��g��+H2��g��=CO��g��+H2O��g����H=+41.0kJ/mol��
�ʴ�Ϊ��CO2��g��+H2��g��=CO��g��+H2O��g����H=+41.0kJ/mol��
��2��0.4molҺ̬�º�0.8mol Һ̬H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ�������������Ȼ�ѧ����ʽ�����壬1mol�º�˫��ˮ֮�䷴Ӧ��ų�641.75kJ������������N2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-641.75 kJ?mol-1���ʴ�Ϊ��N2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-641.75 kJ?mol-1����3���⣺��̼��xmol��������Ϊ��0.2-x��mol����
C��s��+O2��g���TCO2��g����H=-393.5kJ/mol
1 393.5kJ
x 393.5xkJ
2H2��g��+O2��g���T2H2O��g����H=-483.6kJ/mol
2 483.6kJ
��0.2-x��mol 241.8��0.2-x��kJ
����393.5xkJ+241.8��0.2-x��kJ=63.53kJ��
���x=0.1mol����̿����H2�����ʵ���֮��Ϊ0.1mol��0.1mol=1��1��
�ʴ�Ϊ��1��1��
�ڼ��ᣨg��=CO2 ��g��+H2��g����H2=-7.0kJ/mol��
��̬CO2����̬H2 ������̬CO����̬H2O�Ļ�ѧ����ʽΪCO2��g��+H2��g��=CO��g��+H2O��g�����Ը��ݢ�-�ڵõ���
���ԡ�H=34.0kJ/mol-��-7.0kJ/mol��=+41.0kJ/mol����CO2��g��+H2��g��=CO��g��+H2O��g����H=+41.0kJ/mol��
�ʴ�Ϊ��CO2��g��+H2��g��=CO��g��+H2O��g����H=+41.0kJ/mol��
��2��0.4molҺ̬�º�0.8mol Һ̬H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ�������������Ȼ�ѧ����ʽ�����壬1mol�º�˫��ˮ֮�䷴Ӧ��ų�641.75kJ������������N2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-641.75 kJ?mol-1���ʴ�Ϊ��N2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-641.75 kJ?mol-1����3���⣺��̼��xmol��������Ϊ��0.2-x��mol����
C��s��+O2��g���TCO2��g����H=-393.5kJ/mol
1 393.5kJ
x 393.5xkJ
2H2��g��+O2��g���T2H2O��g����H=-483.6kJ/mol
2 483.6kJ
��0.2-x��mol 241.8��0.2-x��kJ
����393.5xkJ+241.8��0.2-x��kJ=63.53kJ��
���x=0.1mol����̿����H2�����ʵ���֮��Ϊ0.1mol��0.1mol=1��1��
�ʴ�Ϊ��1��1��
���������⿼���йط�Ӧ�ȵļ��㣬��ȷ�Ȼ�ѧ��Ӧ����ʽ�����ʵ����������Ĺ�ϵ���ɽ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
�����Ŀ
 ��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ����ͨ������·�ߺϳ�
��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ����ͨ������·�ߺϳ�


 ��XΪ±��ԭ�ӣ�
��XΪ±��ԭ�ӣ� �����ʣ���������һ�����ϣ�
�����ʣ���������һ�����ϣ�