��Ŀ����
ijͬѧ�����һ���״�ȼ�ϵ�أ����øõ�ص��200mL����Ũ��NaCl��CuSO4�����Һ����װ����ͼ��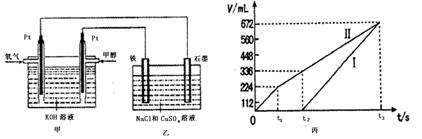
��1��д������ͨ��״���һ���ĵ缫��Ӧʽ ��
��2������������������������������ʱ��仯�Ĺ�ϵ���ͼ��ʾ����������ѻ���ɱ�״���µ��������д����t1��,ʯī�缫�ϵĵ缫��Ӧʽ ��ԭ�����Һ��NaCl�����ʵ���Ũ��Ϊ mol/L��CuSO4�����ʵ���Ũ��Ϊ mol/L����������Һ������䣩
��3�������t3ʱ������ˮ������Ϊ g��
��1��CH3OH-6e��+8OH��=CO32��+6H2O��2�֣�
��2��4OH--4e��=O2��+2H2O ��2�֣� 0.1��2�֣� 0.1��2�֣�
(3)0.72 ��2�֣�
����

��ϰ��ϵ�д�
�����Ŀ


 ��2012?������һģ����Դ��ȱ������������ٵ��ش����⣮�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ����
��2012?������һģ����Դ��ȱ������������ٵ��ش����⣮�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ����

