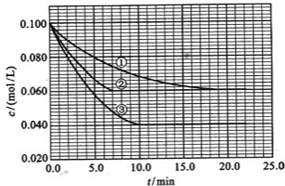
��Ŀ����
������Ԫ��Q��R��T�� W��Ԫ�����ڱ��е�λ������ͼ��ʾ������T��������������������������ȣ���ش��������⣺

(1)T��ԭ�ӽṹʾ��ͼΪ___��
(2)Ԫ�صķǽ�����Ϊ��ԭ�ӵĵõ�����������Q____W(�ǿ�ڡ������ڡ�)��
(3)W�ĵ�����������������ˮ����Ũ��Һ�����ܷ�����Ӧ�������������ʣ�����һ�������壬��Ӧ�Ļ�ѧ����ʽΪ________��
(4)ԭ��������R��1��Ԫ����һ���⻯���ֽܷ�Ϊ������һ���⻯��˷ֽⷴӦ�Ļ�ѧ����ʽ��____��
(2)Ԫ�صķǽ�����Ϊ��ԭ�ӵĵõ�����������Q____W(�ǿ�ڡ������ڡ�)��
(3)W�ĵ�����������������ˮ����Ũ��Һ�����ܷ�����Ӧ�������������ʣ�����һ�������壬��Ӧ�Ļ�ѧ����ʽΪ________��
(4)ԭ��������R��1��Ԫ����һ���⻯���ֽܷ�Ϊ������һ���⻯��˷ֽⷴӦ�Ļ�ѧ����ʽ��____��
(1)
(2)����
(3)S+2H2SO4(Ũ) 3SO2��+2H2O
3SO2��+2H2O
(4)2H2O2 2H2O+O2��
2H2O+O2��

(2)����
(3)S+2H2SO4(Ũ)
 3SO2��+2H2O
3SO2��+2H2O (4)2H2O2
 2H2O+O2��
2H2O+O2��
��ϰ��ϵ�д�
 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
�����Ŀ
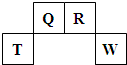 ��2009?������������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
��2009?������������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
 ������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
 ������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ������ͼ��ʾ������Ԫ��T��������������������������ȣ���ش��������⣺
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ������ͼ��ʾ������Ԫ��T��������������������������ȣ���ش��������⣺
 ��2013?��خ��ģ�⣩������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ������ƶ���ȷ���ǣ�������
��2013?��خ��ģ�⣩������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ������ƶ���ȷ���ǣ�������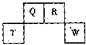 ��2012?������һģ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺
��2012?������һģ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺