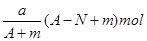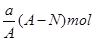��Ŀ����
��12�֣���ش��й������������壺H2��O2��NO��NH3��NO2���� �⡣
I.��1������ȡO2�ķ���װ����ͬ���ǣ�______________ֻ����һ�ַ����ռ�����______________________________
��2�����������Ʊ���Ӧ��,��һ��Ӧ��ԭ������������Ӧ�����ܹ�Ϊͬһ���ͣ���д��ʵ������ȡ������ķ���ʽ_________________________________
II. ����ͼװ�ý�����Ȫʵ�飨ͼ�мг�װ�þ�����ȥ����

��1������ͼ1װ�ý�����Ȫʵ�飬�ϲ���ƿ��װ������İ���������ˮ����IJ�����_____________________��ʵ���ԭ����______________________________________
��2������ͼ2��װ�ã����һ��˵��������Ȫ�ķ���___________________________
III������ͬһ��ƿ�ֱ�����������壺�� HCl �� NH3�� NO2������Ȫʵ�飬ʵ�����ƿ����ҺҺ��ĸ߶ȹ�ϵΪ____________________________ ������ź͡�>,<��=����ʾ����ͬ����������Һ���ʵ���Ũ�ȴ�С��ϵΪ ____________________________ ��
I.��1������ȡO2�ķ���װ����ͬ���ǣ�______________ֻ����һ�ַ����ռ�����______________________________
��2�����������Ʊ���Ӧ��,��һ��Ӧ��ԭ������������Ӧ�����ܹ�Ϊͬһ���ͣ���д��ʵ������ȡ������ķ���ʽ_________________________________
II. ����ͼװ�ý�����Ȫʵ�飨ͼ�мг�װ�þ�����ȥ����

��1������ͼ1װ�ý�����Ȫʵ�飬�ϲ���ƿ��װ������İ���������ˮ����IJ�����_____________________��ʵ���ԭ����______________________________________
��2������ͼ2��װ�ã����һ��˵��������Ȫ�ķ���___________________________
III������ͬһ��ƿ�ֱ�����������壺�� HCl �� NH3�� NO2������Ȫʵ�飬ʵ�����ƿ����ҺҺ��ĸ߶ȹ�ϵΪ____________________________ ������ź͡�>,<��=����ʾ����ͬ����������Һ���ʵ���Ũ�ȴ�С��ϵΪ ____________________________ ��
I����1��NH3�� NO��NH3��NO2��4�֣�
��2��2NH4Cl��Ca(OH)2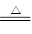 CaCl2��2NH3��+2H2O ��2�֣�
CaCl2��2NH3��+2H2O ��2�֣�
II����1����ֹˮ�У�������ͷ�ι��е�ˮ����1�֣�
���������ܽ���ˮ����ʹ��ƿ������ѹǿѸ�ټ�С����1�֣�
��2�����ӣ����֣�����ë���ȣ�����ƿ���� ��1�֣�
III���٣��ڣ��� (1��) �٣��ڣ��� ��2�֣�
��2��2NH4Cl��Ca(OH)2
 CaCl2��2NH3��+2H2O ��2�֣�
CaCl2��2NH3��+2H2O ��2�֣�II����1����ֹˮ�У�������ͷ�ι��е�ˮ����1�֣�
���������ܽ���ˮ����ʹ��ƿ������ѹǿѸ�ټ�С����1�֣�
��2�����ӣ����֣�����ë���ȣ�����ƿ���� ��1�֣�
III���٣��ڣ��� (1��) �٣��ڣ��� ��2�֣�
I����1��ѡ������ķ���װ����Ҫ���������ʵ�״̬����Ӧ�������������ɹ�̬MnO2����KClO3�ķֽ⣬��������:2KClO3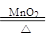 2KCl��3O2��������ʵ������ȡ�����ķ���װ����ͬ��Ca(OH)2+2NH4Cl
2KCl��3O2��������ʵ������ȡ�����ķ���װ����ͬ��Ca(OH)2+2NH4Cl CaCl2��2NH3����2H2O
CaCl2��2NH3����2H2O
NO���������ܹ��棬ֻ������ˮ���ռ�����NH3��NO2������ˮ����ˮ��Ӧ����������ˮ���ռ�����ֻ���õ��������ռ�
��2������������ȡ���Ϊ������ԭ��Ӧ��Ca(OH)2+2NH4Cl CaCl2��2NH3����2H2O
CaCl2��2NH3����2H2O
II.��1����ֹˮ�У�������ͷ�ι��е�ˮ�����ڰ��������ܽ���ˮ����ʹ��ƿ������ѹǿѸ�ټ�С���ձ��е�ˮ�Ϳ�����ƿ���γ���Ȫ
��2�����õķ���ֻҪ���γ�ѹǿ����γ���Ȫ��������ӣ����֣�����ë���ȣ�����ƿ����
III������HCl��NH3����������ˮ����������ƿ������Һռ������3NO2��H2O=2HNO3+NO������Һֻ��ռ��ƿ�ݻ���2/3
��ͬ��ͬѹ�£�������������ͬ�����ʵ�����ͬ�����γ���Һ�������Ϊ3:3:2�����γ���Һ�����ʵ����ʵ�����ҲΪ3:3:2����������Һ���ʵ���Ũ�Ⱦ���ͬ
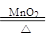 2KCl��3O2��������ʵ������ȡ�����ķ���װ����ͬ��Ca(OH)2+2NH4Cl
2KCl��3O2��������ʵ������ȡ�����ķ���װ����ͬ��Ca(OH)2+2NH4Cl CaCl2��2NH3����2H2O
CaCl2��2NH3����2H2ONO���������ܹ��棬ֻ������ˮ���ռ�����NH3��NO2������ˮ����ˮ��Ӧ����������ˮ���ռ�����ֻ���õ��������ռ�
��2������������ȡ���Ϊ������ԭ��Ӧ��Ca(OH)2+2NH4Cl
 CaCl2��2NH3����2H2O
CaCl2��2NH3����2H2OII.��1����ֹˮ�У�������ͷ�ι��е�ˮ�����ڰ��������ܽ���ˮ����ʹ��ƿ������ѹǿѸ�ټ�С���ձ��е�ˮ�Ϳ�����ƿ���γ���Ȫ
��2�����õķ���ֻҪ���γ�ѹǿ����γ���Ȫ��������ӣ����֣�����ë���ȣ�����ƿ����
III������HCl��NH3����������ˮ����������ƿ������Һռ������3NO2��H2O=2HNO3+NO������Һֻ��ռ��ƿ�ݻ���2/3
��ͬ��ͬѹ�£�������������ͬ�����ʵ�����ͬ�����γ���Һ�������Ϊ3:3:2�����γ���Һ�����ʵ����ʵ�����ҲΪ3:3:2����������Һ���ʵ���Ũ�Ⱦ���ͬ

��ϰ��ϵ�д�
�����Ŀ
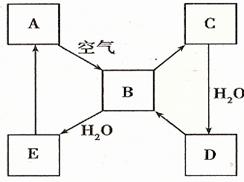
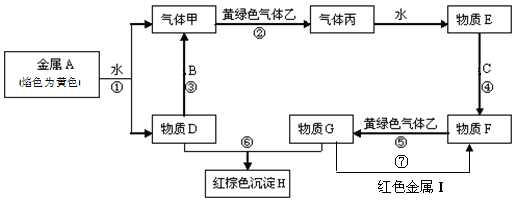
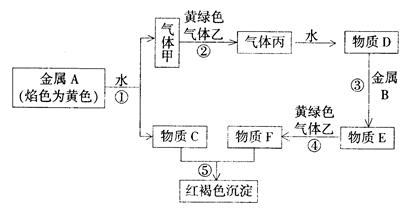
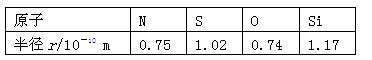
 ��ԭ��������Ϊ
��ԭ��������Ϊ ����
����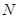 �����ӣ�����
�����ӣ����� ԭ�����
ԭ����� ���ӣ���
���ӣ��� ���������ӵ����ʵ����ǣ� ��
���������ӵ����ʵ����ǣ� ��