��Ŀ����
��.��ѧʵ������ȡ�Ȼ�������ķ���֮һ�ǽ�Ũ�������Ũ�����У������ͼ����ѡ�����������ڷ����ڻ����ø÷����Ʊ����ռ������Ȼ��������װ�ü�ͼ������ͼ�б��������Լ���(�������ظ�ʹ�ã��̶�װ�ò��ػ���)


��.ʵ�����Ʊ������������������ʵ�鲽�����£�ȡ�����ྻ����м������20%��30%��ϡ������Һ����50�桫80��ˮԡ�м��������ٲ������ݣ�����Һ���ȹ��ˣ���Һ�����Թ��У������������Թܿڣ����á���ȴһ��ʱ����ռ���Ʒ��
(1)д����ʵ���Ʊ����������Ļ�ѧ����ʽ��________________________________.
(2)������Һ��ϡ�ᵼ�� ______________________________________��
(3)����ˮԡ���ȵ�ԭ���� __________________________________��
(4)��Ӧʱ��м������Ŀ����(�����ӷ���ʽ��ʾ) _______________________��
(5)��Һ���ȹ��˵�ԭ����_____________________________�������Թܿڵ�Ŀ����__________________________��
(6)���á���ȴһ��ʱ������Թ��й۲쵽�������� ________________________________________��
(1)д����ʵ���Ʊ����������Ļ�ѧ����ʽ��________________________________.
(2)������Һ��ϡ�ᵼ�� ______________________________________��
(3)����ˮԡ���ȵ�ԭ���� __________________________________��
(4)��Ӧʱ��м������Ŀ����(�����ӷ���ʽ��ʾ) _______________________��
(5)��Һ���ȹ��˵�ԭ����_____________________________�������Թܿڵ�Ŀ����__________________________��
(6)���á���ȴһ��ʱ������Թ��й۲쵽�������� ________________________________________��
I.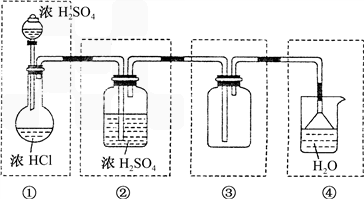

II.(1)Fe��H2SO4(ϡ)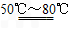 FeSO4��H2��
FeSO4��H2��
(2)��Ӧ�������������ھ�������
(3)�������¶�
(4)Fe��2Fe3��===3Fe2��
(5)����FeSO4����ʧ����ֹ���������Թܽ�Fe2������ΪFe3��
(6)��dz��ɫ��������
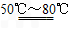 FeSO4��H2��
FeSO4��H2��(2)��Ӧ�������������ھ�������
(3)�������¶�
(4)Fe��2Fe3��===3Fe2��
(5)����FeSO4����ʧ����ֹ���������Թܽ�Fe2������ΪFe3��
(6)��dz��ɫ��������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


 5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��
5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��
 NaC1O3+3H2��
NaC1O3+3H2�� HC1O+H++C1��
HC1O
HC1O+H++C1��
HC1O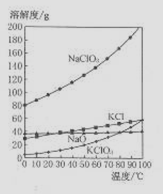
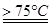 5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��
5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��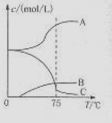
 NaC1O3+3H2��
NaC1O3+3H2�� HC1O+H++C1�� HC1O
HC1O+H++C1�� HC1O