��Ŀ����
����Ŀ����15�֣�ijʵ��С������ͼ��ʾװ���Ʊ�һ�����ױ��������������ױ����������ױ�����

��Ӧԭ����
ʵ���п����õ������ݣ�

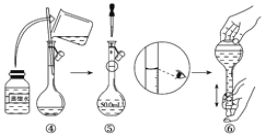
ʵ�鲽�裺��Ũ������Ũ���ᰴ�����1��3���ƻ����Һ�������ᣩ��40 mL��
��������ƿ�м���13g�ױ����ӷ�������ͼ��ʾװ��ҩƷ������������
��������ƿ�м�����
�������¶�ԼΪ50������Ӧ��Լ10 min������ƿ���д�������ɫ��״Һ����֣�
�������һ�����ױ������ᴿ���յõ�������һ�����ױ���15 g��
��ش��������⣺
��1��ʵ��ǰ��Ҫ������ƿ�м�������________��Ŀ����____________________��
��2�������ܵ�������_________����ȴˮ�������ܵ�_______������a������b�����˽��롣
��3������A��������________ ��ʹ�ø�����ǰ������еIJ�����_________________��
��4�����뷴Ӧ�����ķ������£�

���У�����1������Ϊ________������2����IJ��������оƾ��ơ��¶ȼơ���ƿ��ţ�ǹܣ�β�ӹܣ���________________��_________________��
��5����ʵ����һ�����ױ��IJ���Ϊ________���������С�����һλ���֣���
���𰸡���1����ʯ�������Ƭ����1�֣� ��ֹ���У�1�֣�
��2������������1�֣� a��1�֣�
��3����Һ©����1�֣� ����Ƿ�©Һ��1�֣�
��4����Һ��2�֣� ������ƿ��2�֣� �����ܣ�2�֣�
��5��77.5%��3�֣�
�������������������1������Һ�����������У�����ʵ��ǰ��Ҫ������ƿ�м���������ʯ�������Ƭ����Ŀ���Ƿ�ֹ���С�
��2����Ϊ��Ӧ��ױ���HNO3�ӷ������������ܵ���������ů������������ˮ������ʱ������Ч���ã�������ȴˮ�������ܵ�a�˽��롣
��3������װ��ͼ��֪������A�������Ƿ�Һ©����ʹ�÷�Һ©��ǰ������еIJ����ǣ�����Ƿ�©Һ��
��4������1�ѻ��Һ����Ϊ�л��������������������1Ϊ��Һ������2�ѻ��ܵ������л�����룬Ϊ������Ҫ��������������ƿ�������ܡ�
��5��13g�ױ������Ͽ����������ױ�Ϊ��13g��92g/mol��137g.mol=19.36g����һ�����ױ��IJ���Ϊ��15g��19.36g��100%=77.5%

����Ŀ��ͨ������������������������ˮ�����ٷ�Һ�ŷŶԻ�������Ⱦ��ͬʱ����K2Cr2O7��ʵ���ҶԺ�����Һ(����Cr3+��Fe3+��K+��SO42����NO3��������Cr2O72��)�����������ù������£�

��֪����Cr(OH)3 + OH�� = CrO2�� + 2H2O��
��2CrO2�� + 3H2O2 + 2OH�� = 2CrO42�� + 4H2O��
��H2O2�����������¾��л�ԭ�ԣ��ܽ�+6��Cr��ԭΪ+3��Cr��
��1����ͼ����KOH��������250mL 6 mol��L��1 KOH��Һ�Ĺ���ʾ��ͼ��

������۲�ͼʾ�жϣ����в���ȷ�IJ�����(�����)_____________________��
����������250 mL��Һ�����������(������)_________________��
�������ͼʾ�IJ���������Һ�������Ƶ���ҺŨ�Ƚ�________(�ƫ��ƫС��)��
(2)��Һ���ữǰ�����м��ȵ�Ŀ����____________________________����ԡ�����˺�Ӧ��������ˮϴ��K2Cr2O7����Ŀ����_______��
��3���±���������ʵ��ܽ�����ݣ�
���� | 0�� | 20�� | 40�� | 60�� | 80�� | 100�� |
KCl | 28.0 | 34.2 | 40.1 | 45.8 | 51.3 | 56.3 |
K2SO4 | 7.4 | 11.1 | 14.8 | 18.2 | 21.4 | 24.1 | K2Cr2O7 | 4.7 | 12.3 | 26.3 | 45.6 | 73.0 | 102.0 |
KNO3 | 13.9 | 31.6 | 61.3 | 106 | 167 | 246.0 |
�����ܽ�����ݣ�����������������Ϊ��________________��________________��
��ȡ��Ʒ�ظ��������2.000g���250mL��Һ��ȡ��25.00mL����ƿ�У�����10mL 2mol��L��1H2SO4�������⻯��(���Ļ�ԭ����ΪCr3+�������ڰ���5min��Ȼ�����100mLˮ�� ����3mL����ָʾ������0.1200 mol��L��1Na2S2O3����Һ�ζ�(I2+2S2O32��=2I��+S4O62��)��
��д���ظ���������⻯�Ƶ����ӷ���ʽ_______________________��
�ڵζ��յ������Ϊ_________________________��
����ʵ���й���ȥNa2S2O3����Һ30.00mL�����ò�Ʒ�е��ظ���صĴ��� Ϊ_________(�������������������ʲ����뷴Ӧ)��
�����ζ�����ʹ��ǰδ��Na2S2O3����Һ��ϴ����õ��ظ���صĴ��Ƚ�_____________(�ƫ�ߡ�����ƫ�͡����䡱)��
����Ŀ����ͼ��ʾ�����ձ���ʢ�к�ˮ���������б���ʴ���ٶ��ɿ쵽����˳��Ϊ

A. �ڢ٢ۢܢݢ� B. �ݢܢڢ٢ۢ� C. �ݢڢܢ٢ۢ� D. �ݢۢڢܢ٢�
����Ŀ������ʵ����ƻ������ȷ���ǣ� ��
ѡ�� | ʵ��Ŀ�� | ʵ����� |
A | ��ȥ����������������Ĥ | ���������� NaOH ��Һ��ϴ�� |
B | ��ˮ���ռ� KMnO4 �ֽ������ O2 | ��Ϩ��ƾ��ƣ����Ƴ����� |
C | ������Һ���Ƿ��� Fe2�� | ����Һ�е�����ˮ���ٵμ� KSCN ��Һ |
D | ֤�� H2SO4 ���Ա� H2CO3 ǿ | ��ϡ H2SO4 ���� NaHCO3 ��Һ |

