��Ŀ����
��8�֣���һ�������£�ijЩ��ѧ��Ӧ������ͼ��ܱ�ʾ������Ҫ��ش��������⣺

��1����A��C��D��Ϊ�������ʣ���A�Ļ��ϼ۽���C��D֮�䣬D����Ư���ԣ�д���÷�Ӧ�����ӷ���ʽ ��
��2����ͨ�����ķ�����û���ɫ����C����÷�Ӧ�����ӷ���ʽΪ
��3����C��D��Ϊ�����Ҿ���ʹ����ʯ��ˮ����ǣ�д������������ͼҪ��Ļ�ѧ����ʽ
��4����AΪ��ɫ�������ʣ�BΪ�ڶ�����ij��Ԫ�ص�����������ˮ�����ϡ��Һ��
д������������ͼ��ϵ�����ӷ���ʽ������ ��������������
(1) Cl2+2OH�� = Cl��+ClO��+H2O��
��2��2Cl��+2H2O Cl2+H2+2OH����
Cl2+H2+2OH����
��3��C+2H2SO4��Ũ�� CO2+2SO2+2H2O��
CO2+2SO2+2H2O��
��4��3Cu+8H++2NO3�� =3Cu2++2NO+4H2O��
��������
�����������1��D����Ư���ԣ���A��C��D������Ԫ�أ�������Cl2+2NaOH=NaCl+NaClO+H2O��
��2����ⱥ��ʳ��ˮ�����������ơ�������������
��3��C��D����ʹ����ʯ��ˮ����ǣ�C��DӦΪ������̼�Ͷ�������Ӧ����ʽΪC+2H2SO4��Ũ�� CO2+2SO2+2H2O ��
CO2+2SO2+2H2O ��
��4����ɫ����Ϊͭ���ڶ�����Ԫ������������Ӧˮ����ϡ��Һ����ΪH3BO3��H2CO3��HNO3��ͭ����ϡ���ᷴӦ�����ӷ���ʽΪ3Cu+8H++2NO3��=3Cu2++2NO��+4H2O��
���㣺���ƶ�
����������֪ʶ��Ƚϴ�˼��������

 ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д� ��R1��R2��R3����������
��R1��R2��R3����������





 ��R1��R2��R3����������
��R1��R2��R3����������

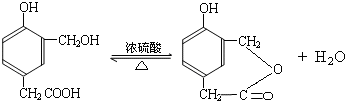
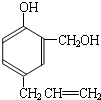
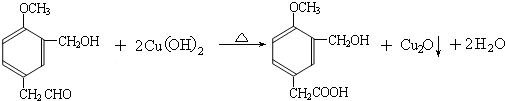
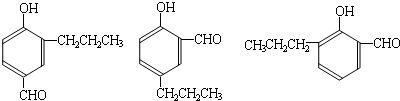
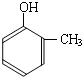 ���ĺϳ�
���ĺϳ�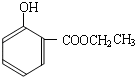 ·�ߣ��úϳ�·������ͼ��ʾ����ע����Ӧ��������
·�ߣ��úϳ�·������ͼ��ʾ����ע����Ӧ��������

 ���䷴Ӧ����ʽΪ
���䷴Ӧ����ʽΪ


 AlN+3CO
AlN+3CO