��Ŀ����
19��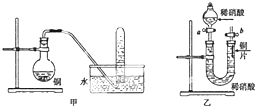 ��ͼ�Ǽס�����λͬѧ̽��ͭ��ϡ���ᣮ��Ӧ��ԭ�����ʵ��װ��ͼ����ش��������⣺
��ͼ�Ǽס�����λͬѧ̽��ͭ��ϡ���ᣮ��Ӧ��ԭ�����ʵ��װ��ͼ����ش��������⣺��1��д��ͭ��ϡ���ᷴӦ�Ļ�ѧ����ʽ3Cu+8HNO3=3Cu��NO3��2+2NO��+4H2O��
��2��ʵ��װ�ü���ƿ���к���ɫ�����ԭ���ǣ�д��ѧ����ʽ����2NO+O2=2NO2��
��3���ֱ�������ͭƬ��������������Ũ�����ϡ���ᷴӦ�����õ�����Һǰ�߳���ɫ�����߳���ɫ��ijͬѧ������������Cu2+��Ũ�Ȳ�������ģ���ͬ�����ֿ�����
��ͬ�� ����ͬ���ͬ�⣩��ԭ����ͭƬ������ͬ����Һ�������ͬ�����ɵ�Cu2+Ũ����ͬ��
���� ��1��Cu��ϡ���ᷴӦ��������ͭ��NO��ˮ��
��2�����ɵ�һ�������ܱ���������Ϊ����ɫ�Ķ���������
��3����������ͭƬ�ֱ���������������Ũ���������ϡ���ᷴӦʱͭȫ��ת��Ϊ���ӣ�
��� �⣺��1��Cu��ϡ���ᷴӦ��������ͭ��NO��ˮ����Ӧ�Ļ�ѧ����ʽΪ3Cu+8HNO3=3Cu��NO3��2+2NO��+4H2O��
�ʴ�Ϊ��3Cu+8HNO3=3Cu��NO3��2+2NO��+4H2O��
��2��һ�������ܱ���������Ϊ����ɫ�Ķ�����������2NO+O2=2NO2���ʴ�Ϊ��2NO+O2=2NO2��
��3������������ͭƬ�ֱ���������������Ũ���������ϡ���ᷴӦ��������Һ��Cu2+��Ũ�Ȼ�����ȣ���ɫ������ͬ����������Cu2+Ũ�Ȳ�������ģ�����Һ�ʡ���ɫ����������Һ��Cu2+��NO2����Ľ����
�ʴ�Ϊ����ͬ�⣻ͭƬ������ͬ����Һ�������ͬ�����ɵ�Cu2+Ũ����ͬ��
���� ���⿼����Ũ���ᡢϡ����ֱ���ͭ��Ӧ��ʵ�飬����ʵ��̽������Ŀ���Ѷ��еȣ�ע��Ũ�����ϡ������ͭ��Ӧ����IJ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
18���������������Һ������Ȫʵ�飬����Һ�岻�ܳ�����ƿ���ǣ�������
| A�� | CO2��NaOH��Һ | B�� | NO2��ˮ | C�� | NH3��ˮ | D�� | Cl2��NaOH��Һ |
10��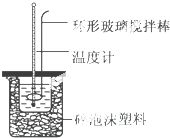 ʵ������50mL 0.50mol•L-1���ᡢ50mL 0.55mol•L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ��±��е�����
ʵ������50mL 0.50mol•L-1���ᡢ50mL 0.55mol•L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ��±��е�����
�����������
��1��ʵ��ʱ������ͭ˿��������滷�β����������������Cu���ȿ죬������ʧ��
��2���ڲ�����ȷ��ǰ���£�����к��Ȳⶨȷ�ԵĹؼ������װ�õı���Ч����
��3�������ϱ����������ݽ��м��㣬���ʵ���õ��к��ȡ�H=-56.8 kJ/mol[�����NaOH��Һ���ܶȰ�1g•cm-3���㣬��Ӧ������Һ�ı����ݣ�c����4.18J•��g•�棩-1����]�������0.5mol/L��������NaOH�������ʵ�飬��ʵ���в�õġ��к��ȡ���ֵ��ƫ���ƫ����ƫС�����䡱����
��4����ijͬѧ��������װ����ʵ�飬��Щ�������淶����ɲ���к��ȵ���ֵƫ�ͣ�����������ܵ�ԭ����ABE��
A������������¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ�
B������Ͳ�е�����������Һ����С�ձ�ʱ�����ٻ�
C������ʵ��ĵ������½ϸ�
D������ȡ����ʱ���Ӷ���
E�����ձ��ĸǰ��м�С��̫��
 ʵ������50mL 0.50mol•L-1���ᡢ50mL 0.55mol•L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ��±��е�����
ʵ������50mL 0.50mol•L-1���ᡢ50mL 0.55mol•L-1 NaOH��Һ����ͼ��ʾװ�ã����вⶨ�к��ȵ�ʵ�飬�õ��±��е�����| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�� ��t2/�� | |
| ���� | NaOH��Һ | ||
| 1 | 20.2 | 20.3 | 23.7 |
| 2 | 20.3 | 20.5 | 23.8 |
| 3 | 21.5 | 21.6 | 24.9 |
��1��ʵ��ʱ������ͭ˿��������滷�β����������������Cu���ȿ죬������ʧ��
��2���ڲ�����ȷ��ǰ���£�����к��Ȳⶨȷ�ԵĹؼ������װ�õı���Ч����
��3�������ϱ����������ݽ��м��㣬���ʵ���õ��к��ȡ�H=-56.8 kJ/mol[�����NaOH��Һ���ܶȰ�1g•cm-3���㣬��Ӧ������Һ�ı����ݣ�c����4.18J•��g•�棩-1����]�������0.5mol/L��������NaOH�������ʵ�飬��ʵ���в�õġ��к��ȡ���ֵ��ƫ���ƫ����ƫС�����䡱����
��4����ijͬѧ��������װ����ʵ�飬��Щ�������淶����ɲ���к��ȵ���ֵƫ�ͣ�����������ܵ�ԭ����ABE��
A������������¶Ⱥ��¶ȼ�û����ˮ��ϴ�ɾ�
B������Ͳ�е�����������Һ����С�ձ�ʱ�����ٻ�
C������ʵ��ĵ������½ϸ�
D������ȡ����ʱ���Ӷ���
E�����ձ��ĸǰ��м�С��̫��
7����ѧ�뻷������������أ�����������ȷ���ǣ�������
| A�� | ��ɫ��ѧ�ĺ�����Ӧ�û�ѧԭ���Ի�����Ⱦ�������� | |
| B�� | ������ˮʱ����������Ϊ��������ˮ����ɱ������ | |
| C�� | PM2.5��2.5�����µ�ϸ�������Ҫ���Ի�ʯȼ�ϵ�ȼ�� | |
| D�� | ij��ˮ��Ʒ�ɼ������һ��ʱ�䣬pH��4.68��Ϊ4.28������Ϊˮ���ܽ��˽϶��CO2 |
14�����ʵ�����ͬ�������л�����ȼ�պ����������ǣ�������
| A�� | C2H2 | B�� | C2H6O | C�� | C4H6 | D�� | C2H4 |
4����25��ʱ����0.2mol CH3COONa�����0.1mol HCl����ͬʱ�ܽ���ͬһ�ձ���ˮ�У��Ƶ�1L��Һ��������Һ��c��CH3COO-����c��Cl-�����������жϲ���ȷ���ǣ�������
| A�� | ����Һ��pHС��7 | B�� | c��CH3COOH��+c��CH3COO-��=0.20 mol•L-1 | ||
| C�� | c��CH3COO-��+c��OH-��=0.10 mol•L-1 | D�� | c��CH3COOH����c��CH3COO-�� |
11��ˮú������Ҫȼ�Ϻͻ���ԭ�ϣ�����ˮ����ͨ�����ȵ�̿���Ƶã�

C��s��+H2O��g��?CO ��g��+H2 ��g����H=+131.3kJ•mol-1
��1���÷�Ӧ��ƽ�ⳣ��K���¶ȵ����߶���������С�����䣩��
��2��������Ӧ�ﵽƽ�������H2O��g����������C��s�������ʵ�����С������С�����䣩��
��3������˵��һ�����ж����Ϸ�Ӧ��ƽ�����BD
A������1mol H2O��g��ͬʱ����1mol H2
B�������ڻ�����������������
C��H2O��g����CO ��g����H2 ��g�����������Ũ�ȱ�Ϊ1��1��1
D�����º���ʱ�����������ѹǿ����
��4��������Ӧ��t0ʱ�̴ﵽƽ�⣬��t1ʱ�̸ı�ijһ����������Ӧ���ʣ���������ʱ��ı仯��ͼ1��ʾ��
�����Ӧ�ı�ţ�
�����������b��
�ڽ����¶�f��
��5��һ���¶��£����������о�������������Ӧ����������̿�������������ʵ����ʵ���Ũ�ȼ����淴Ӧ���ʹ�ϵ���±���ʾ������д���Т٢���Ӧ�Ŀո�
��6 ����ͼ2����������ȼ������Դ���ö��Ե缫���200ml 1mol/Lʳ��ˮ�����һ��ʱ����ռ�����״���µ�����4.48L���������Һ������䣩��
�ٵ�����Һ��pH=14����������������������Һ��Ӧ��
�������������������ڱ�״������3.36 L��

C��s��+H2O��g��?CO ��g��+H2 ��g����H=+131.3kJ•mol-1
��1���÷�Ӧ��ƽ�ⳣ��K���¶ȵ����߶���������С�����䣩��
��2��������Ӧ�ﵽƽ�������H2O��g����������C��s�������ʵ�����С������С�����䣩��
��3������˵��һ�����ж����Ϸ�Ӧ��ƽ�����BD
A������1mol H2O��g��ͬʱ����1mol H2
B�������ڻ�����������������
C��H2O��g����CO ��g����H2 ��g�����������Ũ�ȱ�Ϊ1��1��1
D�����º���ʱ�����������ѹǿ����
��4��������Ӧ��t0ʱ�̴ﵽƽ�⣬��t1ʱ�̸ı�ijһ����������Ӧ���ʣ���������ʱ��ı仯��ͼ1��ʾ��
�����Ӧ�ı�ţ�
�����������b��
�ڽ����¶�f��
��5��һ���¶��£����������о�������������Ӧ����������̿�������������ʵ����ʵ���Ũ�ȼ����淴Ӧ���ʹ�ϵ���±���ʾ������д���Т٢���Ӧ�Ŀո�
| ������� | c��H2O��/mol•L-1 | c��CO��/mol•L-1 | c��H2��/mol•L-1 | �����������Ƚ� |
| I | 0.06 | 0.60 | 0.10 | ����=���� |
| �� | 0.12 | 0.20 | ��0.6 | ����=���� |
| �� | 0.10 | 0.20 | 0.40 | �ڦ��������� |
�ٵ�����Һ��pH=14����������������������Һ��Ӧ��
�������������������ڱ�״������3.36 L��
8�����з���ʽ��ʾ������һ���Ǵ�������ǣ�������
| A�� | C4H10 | B�� | CH2Cl2 | C�� | C2H4O2 | D�� |  |
9������˵������ȷ���ǣ�������
| A�� | ������״̬�£�1molNa2O2��ȫ�������������ĿΪ3NA��NA���������ӵ�������ֵ�� | |
| B�� | ���������ﲻһ���Ƿǽ������������������һ���ǽ��������� | |
| C�� | �����´�����Ӳ����ܴ�����pH��7�ļ�����Һ�� | |
| D�� | �ڢ�A��Ԫ�ص��⻯���У��ȶ�����õ���е�Ҳ��� |