��Ŀ����
���� 20�֣�
20�֣�
(i) ���з�����Ϊ58�ļ����л����д���������� ���л���Ľṹ��ʽ
���л���Ľṹ��ʽ
��1�������л���Ϊ��������ܵĽṹ��ʽΪ�� �� ��
��2�������л�����һ�ֱ���һԪȩ������ṹ��ʽΪ�� ��
��3�������л���1mol��������������Һ���ÿ�����4molAg�����л���Ľṹ��ʽΪ�� ��
��4�������л������������Ʒ�Ӧ�ų�����������ʹ������Ȼ�̼��Һ��ɫ������л���ṹ��ʽ������ ����ע���ǻ�����˫���ϵ��л��K���ȶ���
��ii����֪���� R-CH2-COOH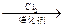
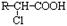 ��
��
�� R-ONa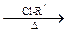 R-O-R�� (R-��R��-��������)��
R-O-R�� (R-��R��-��������)��
��֪ij������һ�־�������������ʳ�����ϣ�������·�ߣ���Ӧ������ȥ�����£�
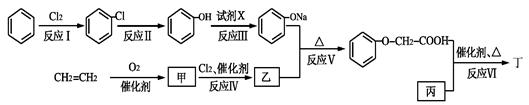
��ش��������⣺
��1����������Է�������Ϊ58��1 mol����ȫȼ�տɲ���3.0 mol CO2��3.0 mol H2O���ұ������в�������Ϊ��״�ṹ������ṹ��ʽ�� ��
��2����Ӧ��������� ����Ӧ���ķ�Ӧ������ ��
��3���ҵĽṹ��ʽ�� ��
��
��Ӧ���Ļ�ѧ����ʽ�� ��
 20�֣�
20�֣�(i) ���з�����Ϊ58�ļ����л����д����������
 ���л���Ľṹ��ʽ
���л���Ľṹ��ʽ��1�������л���Ϊ��������ܵĽṹ��ʽΪ�� �� ��
��2�������л�����һ�ֱ���һԪȩ������ṹ��ʽΪ�� ��
��3�������л���1mol��������������Һ���ÿ�����4molAg�����л���Ľṹ��ʽΪ�� ��
��4�������л������������Ʒ�Ӧ�ų�����������ʹ������Ȼ�̼��Һ��ɫ������л���ṹ��ʽ������ ����ע���ǻ�����˫���ϵ��л��K���ȶ���
��ii����֪���� R-CH2-COOH
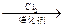
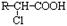 ��
���� R-ONa
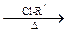 R-O-R�� (R-��R��-��������)��
R-O-R�� (R-��R��-��������)����֪ij������һ�־�������������ʳ�����ϣ�������·�ߣ���Ӧ������ȥ�����£�

��ش��������⣺
��1����������Է�������Ϊ58��1 mol����ȫȼ�տɲ���3.0 mol CO2��3.0 mol H2O���ұ������в�������Ϊ��״�ṹ������ṹ��ʽ�� ��
��2����Ӧ��������� ����Ӧ���ķ�Ӧ������ ��
��3���ҵĽṹ��ʽ��
 ��
����Ӧ���Ļ�ѧ����ʽ�� ��
��20�֣�ÿ��2�֣�
(i)��1��CH3CH2CH2CH3�� CH3CH(CH3)2 ��2��CH3CH2CHO ��3��OHC-CHO ��4��CH2=CH-CH2OH
(ii)��1��CH2=CHCH2OH ��2�����ۣ����Ȼ����������� ȡ����Ӧ ��3��ClCH2COOH

(i)��1��CH3CH2CH2CH3�� CH3CH(CH3)2 ��2��CH3CH2CHO ��3��OHC-CHO ��4��CH2=CH-CH2OH
(ii)��1��CH2=CHCH2OH ��2�����ۣ����Ȼ����������� ȡ����Ӧ ��3��ClCH2COOH

��

��ϰ��ϵ�д�
 �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�
�����Ŀ



 �ۡ�CH�TCH2
�ۡ�CH�TCH2
 ��
��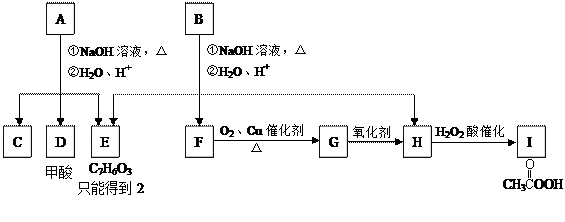

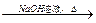 CH3COONa��
CH3COONa��

