��Ŀ����
[��ѧ-��ѧ�뼼��]ú����Ҫ����Դ��Ҳ�ǻ�����������Ҫԭ�ϣ�
��1��úȼ�ղ����ķ���ֱ���ŷŵ������У����ܵ��µĻ�����Ⱦ������______��
��2����ͼ�Ƕ�úȼ�ղ����ķ������г���������������ʾ��ͼ��

�ٷ�����������У���Ҫ��Ӧ�Ļ�ѧ����ʽΪ______��______��
����ú��ֱ������һ�����ʣ�����Ч����úȼ�ղ�����SO2����������______��
��ʯ���ڹ�ҵ�����е���;��______��д��һ����;���ɣ���
��3��ú����______����ӹ����������Եõ�¯ú����ú���ͺͽ�̿��ú���;���______����ӹ��������ɵõ������廯���úҲ�������⻯��ת����ȼ�ͣ��⻯���ı�����______��
�⣺��1��úȼ�ղ����ķ����к��ж����������������������ꣻ�ʴ�Ϊ�����ꣻ
��2������ʯ��ʯ�Ϳ��������������Ƕ��������̼������ɶ�����̼��������ƣ�SO2+CaCO3 CaSO3+CO2��������Ʊ���������Ϊ����ƣ�2CaSO3+O2=2CaSO4��
CaSO3+CO2��������Ʊ���������Ϊ����ƣ�2CaSO3+O2=2CaSO4��
�ʴ�Ϊ��SO2+CaCO3 CaSO3+CO2��2CaSO3+O2=2CaSO4��
CaSO3+CO2��2CaSO3+O2=2CaSO4��
����CaCO3��CaO��Ca��OH��2���ܺ�SO2�������ն����Ƴ�ʯ�࣬���Ϊ�����ʴ�Ϊ��CaCO3��CaO��Ca��OH��2��
��ʯ���������ܡ����������̶����۵Ĺ�ͷ���������ϣ��ʴ�Ϊ���������ϣ�
��3��ú����������Եõ�¯ú����ú���ͺͽ�̿��ú���;�������ɵõ������廯�����ú�к���̼Ԫ�أ���������Ԫ�أ���ȼ�����溬��̼Ԫ�غ���Ԫ�أ�����úҲ�����ü���ת����ȼ�ͣ��ʴ�Ϊ������������ú����Ԫ�غ����������ԭ������̼ԭ�����ı�ֵ��
��������1��úȼ�ղ����ķ����к��ж����������������������ꣻ
��2���ٸ���ͼʾ�ÿ�֪ʯ��ʯ�Ϳ��������������Ƕ��������̼������ɶ�����̼��������ƣ�������Ʊ���������Ϊ����ƣ�
�ڸ���CaCO3��CaO��Ca��OH��2���ܺ�SO2�������ն����Ƴ�ʯ�࣬���Ϊ����
�۸���ʯ���������ܡ����������̶����۵Ĺ�ͷ���������ϵȣ�
��3�����ݹ�ҵ��úת��Ϊ��¯����ú���ͺͽ�̿�ȳ��ø���ķ����Ʊ�����ú�����еõ������廯���ﳣ�÷���ķ����Ʊ�������ú�к���̼Ԫ�أ���������Ԫ�أ���ȼ���Ƕ������Ļ������溬��̼Ԫ�غ���Ԫ�أ�
������������Ҫ������ú���ۺ����ã��漰����������Ĵ��������ն�������������ǽ��Ĺؼ���
��2������ʯ��ʯ�Ϳ��������������Ƕ��������̼������ɶ�����̼��������ƣ�SO2+CaCO3
 CaSO3+CO2��������Ʊ���������Ϊ����ƣ�2CaSO3+O2=2CaSO4��
CaSO3+CO2��������Ʊ���������Ϊ����ƣ�2CaSO3+O2=2CaSO4���ʴ�Ϊ��SO2+CaCO3
 CaSO3+CO2��2CaSO3+O2=2CaSO4��
CaSO3+CO2��2CaSO3+O2=2CaSO4������CaCO3��CaO��Ca��OH��2���ܺ�SO2�������ն����Ƴ�ʯ�࣬���Ϊ�����ʴ�Ϊ��CaCO3��CaO��Ca��OH��2��
��ʯ���������ܡ����������̶����۵Ĺ�ͷ���������ϣ��ʴ�Ϊ���������ϣ�
��3��ú����������Եõ�¯ú����ú���ͺͽ�̿��ú���;�������ɵõ������廯�����ú�к���̼Ԫ�أ���������Ԫ�أ���ȼ�����溬��̼Ԫ�غ���Ԫ�أ�����úҲ�����ü���ת����ȼ�ͣ��ʴ�Ϊ������������ú����Ԫ�غ����������ԭ������̼ԭ�����ı�ֵ��
��������1��úȼ�ղ����ķ����к��ж����������������������ꣻ
��2���ٸ���ͼʾ�ÿ�֪ʯ��ʯ�Ϳ��������������Ƕ��������̼������ɶ�����̼��������ƣ�������Ʊ���������Ϊ����ƣ�
�ڸ���CaCO3��CaO��Ca��OH��2���ܺ�SO2�������ն����Ƴ�ʯ�࣬���Ϊ����
�۸���ʯ���������ܡ����������̶����۵Ĺ�ͷ���������ϵȣ�
��3�����ݹ�ҵ��úת��Ϊ��¯����ú���ͺͽ�̿�ȳ��ø���ķ����Ʊ�����ú�����еõ������廯���ﳣ�÷���ķ����Ʊ�������ú�к���̼Ԫ�أ���������Ԫ�أ���ȼ���Ƕ������Ļ������溬��̼Ԫ�غ���Ԫ�أ�
������������Ҫ������ú���ۺ����ã��漰����������Ĵ��������ն�������������ǽ��Ĺؼ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
����ѧ-��ѧ�뼼����ijǿ���Թ�ҵ��ˮ�к���Fe2+��Fe3+��Cu2+�����ʵ�鷽���ó�������ȥ�����ӣ��õ��ϴ���Cu2+��Һ���й��������������pH���±���
��1���ӱ������ݷ�����Ϊʲô����ֱ�Ӽ�NaOH����ҺpH����9.7����ȥFe3+��Fe2+ ��
��2��ʵ��Ӧ���������ȼ�һ�����Ĵ������ƣ�Ȼ���ٵ�����ҺpH���������Ƶ������� ��
��3��pHӦ���ڵ�ʲô��Χ ��Ϊʲô ��
��4������pH���˵��Լ���
A����������B��̼��þ��C������ͭ��D��ϡ����
���� ��
| �������� | pH | |
| ��ʼ���� | ������ȫ | |
| Fe2+ | 6.3 | 9.7 |
| Cu2+ | 4.7 | 6.7 |
| Fe3+ | 1.9 | 3.2 |
��2��ʵ��Ӧ���������ȼ�һ�����Ĵ������ƣ�Ȼ���ٵ�����ҺpH���������Ƶ�������
��3��pHӦ���ڵ�ʲô��Χ
��4������pH���˵��Լ���
A����������B��̼��þ��C������ͭ��D��ϡ����
����
����ѧ--��ѧ�뼼����
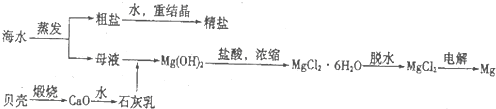
������һ����ı��أ�������Դ�Ŀ��������þ��й�����ǰ����ij�غ�ˮ��pH��7.5��8.6֮�䣬������Ҫ���ӵĺ������±���
��1�����������ǽ��귢չ������һ�ֽϺõĺ�ˮ������������ԭ����ͼ1�������� ���������ӽ���Ĥֻ����������������ͨ����
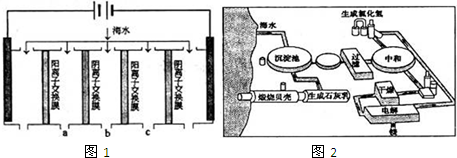
�������ĵ缫��ӦʽΪ ��
�ڵ��һ��ʱ�䣬�����������ˮ������ɷ�ΪCaCO3��Mg��OH��2��д������CaCO3�����ӷ���ʽ ��
�۵�ˮ�ij���Ϊa��b��c�е� ���ڣ�
��2����ͼ2�ǹ�ҵ������þ�����̣�
�ٸ��ﲽ���н��Ȼ�þ��ˮ�Ͼ���ת��Ϊ��ˮ�Ȼ�þ�IJ��������� ��
���������������У�ѭ��ʹ�õ������� ��
��������Ϊ�����˲����ֱ�Ӽ���Mg��OH��2��MgO���ٵ�����ڵ�MgO�ƽ���þ���������Ż��������̣���Ĺ۵��� ���ͬ�⡱��ͬ�⡱���������� ��
������һ����ı��أ�������Դ�Ŀ��������þ��й�����ǰ����ij�غ�ˮ��pH��7.5��8.6֮�䣬������Ҫ���ӵĺ������±���
| �ɷ� | Na+ | K+ | Ca2+ | Mg2+ | Cl- | SO 42- | HCO 3+ | ����/mg?L-1 | 9360 | 83 | 200 | 1100 | 16000 | 1200 | 118 |

�������ĵ缫��ӦʽΪ
�ڵ��һ��ʱ�䣬�����������ˮ������ɷ�ΪCaCO3��Mg��OH��2��д������CaCO3�����ӷ���ʽ
�۵�ˮ�ij���Ϊa��b��c�е�
��2����ͼ2�ǹ�ҵ������þ�����̣�
�ٸ��ﲽ���н��Ȼ�þ��ˮ�Ͼ���ת��Ϊ��ˮ�Ȼ�þ�IJ���������
���������������У�ѭ��ʹ�õ�������
��������Ϊ�����˲����ֱ�Ӽ���Mg��OH��2��MgO���ٵ�����ڵ�MgO�ƽ���þ���������Ż��������̣���Ĺ۵���