��Ŀ����
���ֶ�����Ԫ��A��B��C��D�����ʻ�ṹ��Ϣ���£���Ϣ��ԭ�Ӱ뾶��С��A��B��C��D
��Ϣ������Ԫ��֮���γɵ�ij���ַ��ӵı���ģ�ͼ��������ʣ�

�����������Ϣ�ش��������⣮
��1����CԪ�������ڱ��е�λ��______����д��BC2���ӵĵ���ʽ______��
��AԪ�صĵ��������ʼ�����Ӧ�����ӷ���ʽ______��
����PtΪ�缫��KOHΪ�������Һ�������ֱ�ͨ���Һ�C�ĵ��ʿ����ȼ�ϵ�أ�д����صĵ缫��Ӧʽ������______������______
��2��A���������У�EԪ�صĵ��ʻ�ԭ����ǿ��A��E���ʷ�Ӧ�õ��Ļ�����M��һ����Ҫ�Ļ���ԭ�ϣ���ͼ�ǵ��100ml����M��Һ��װ�ã�X��Y���Ƕ��Ե缫��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����

�ٵ�����Y���ϵĵ缫��Ӧʽ______������Y�缫��Ӧ����ķ�����______��
�ڵ��һ��ʱ����������ռ���112ml��״���µ����壬��ʱ���Һ��PHΪ______��������Һ��������ֲ��䣬�����£�
���𰸡����������ǵ��������������֮һ���ǰ��������������������������Ҫ��Դ��Ҳ������������Ҫ����ɲ��֣�Ӧ��H2O�����ݽṹ����;��֪������CH4������ǿ���������ᣬ������������ɱ����Ӧ��HClO����������Ԫ�طֱ�ΪH��C��O��Cl������ԭ�Ӱ뾶��С˳���֪A��Cl��B��C��C��O��D��H������ԭ�ӵĽṹ�ص��ƶ�Ԫ�������ڱ��е�λ�ã�������ʵ����ʺ�ԭ���ԭ�������⣮
����⣺���ǵ��������������֮һ���ǰ��������������������������Ҫ��Դ��Ҳ������������Ҫ����ɲ��֣�Ӧ��H2O�����ݽṹ����;��֪������CH4������ǿ���������ᣬ������������ɱ����Ӧ��HClO����������Ԫ�طֱ�ΪH��C��O��Cl������ԭ�Ӱ뾶��С˳���֪A��Cl��B��C��C��O��D��H��
��1����C��OԪ�أ�������2�����Ӳ㣬����������Ϊ6��Ӧλ�ڵڶ����ڢ�A�壬B��C�γɵĻ�����ΪCO2��Ϊ���ۻ��������ʽΪ ��
��
�ʴ�Ϊ���ڶ����ڢ�A�壻 ��
��
��Cl2��ˮ��Ӧ����HCl��HClO������HClΪǿ�ᣬ��ȫ���룬HClOΪ���ᣬ
��Ӧ�����ӷ���ʽΪCl2+H2O H++Cl-+HClO��
H++Cl-+HClO��
�ʴ�Ϊ��Cl2+H2O H++Cl-+HClO��
H++Cl-+HClO��
��CH4���л�ԭ�ԣ��ڷ�Ӧ���ױ�������ʧȥ���ӣ���ԭ��صĸ������缫��ӦʽΪCH4-8e-+10OH-=CO32-+7H2O��O2���������ԣ���ԭ��ص�������������ԭ��Ӧ��
�缫��ӦʽΪ2O2+8e-+4H2O=8OH-��
�ʴ�Ϊ��CH4-8e-+10OH-=CO32-+7H2O��2O2+8e-+4H2O=8OH-��
��2����A���������У�EԪ�صĵ��ʻ�ԭ����ǿ����EΪNaԪ�أ��õ������ҺΪNaCl��Һ�����ʱ������ӦΪ2Cl-2e-=Cl2����Cl2����ǿ�����ԣ�����ʪ��ĵ⻯�ص�����ֽ����Y�缫�������飬��������ֽ����ɫ��
�ʴ�Ϊ��2Cl-2e-=Cl2���� ��ʪ��ĵ⻯�ص�����ֽ����Y�缫��������ֽ����ɫ��
�ڵ��ʱ��������ӦΪ��2H2O+2e-=2OH-+H2�����������������ʵ���Ϊ =0.005mol��
=0.005mol��
��n��OH-��=2��H2��=2×0.005mol=0.01mol��c��OH-��= =0.1mol/L��
=0.1mol/L��
����c��OH-��?c��H+��=1×10-14����c��H+��=1×10-13mol/L��
PH=-lg��10-13��=13��
�ʴ�Ϊ��13
���������⿼���Ϊ�ۺϣ��漰Ԫ�ص��ƶϣ����ʵ����ʺ���;�Լ��绯ѧ��֪ʶ��ע��缫����ʽ����д������
����⣺���ǵ��������������֮һ���ǰ��������������������������Ҫ��Դ��Ҳ������������Ҫ����ɲ��֣�Ӧ��H2O�����ݽṹ����;��֪������CH4������ǿ���������ᣬ������������ɱ����Ӧ��HClO����������Ԫ�طֱ�ΪH��C��O��Cl������ԭ�Ӱ뾶��С˳���֪A��Cl��B��C��C��O��D��H��
��1����C��OԪ�أ�������2�����Ӳ㣬����������Ϊ6��Ӧλ�ڵڶ����ڢ�A�壬B��C�γɵĻ�����ΪCO2��Ϊ���ۻ��������ʽΪ
 ��
���ʴ�Ϊ���ڶ����ڢ�A�壻
 ��
����Cl2��ˮ��Ӧ����HCl��HClO������HClΪǿ�ᣬ��ȫ���룬HClOΪ���ᣬ
��Ӧ�����ӷ���ʽΪCl2+H2O
 H++Cl-+HClO��
H++Cl-+HClO���ʴ�Ϊ��Cl2+H2O
 H++Cl-+HClO��
H++Cl-+HClO����CH4���л�ԭ�ԣ��ڷ�Ӧ���ױ�������ʧȥ���ӣ���ԭ��صĸ������缫��ӦʽΪCH4-8e-+10OH-=CO32-+7H2O��O2���������ԣ���ԭ��ص�������������ԭ��Ӧ��
�缫��ӦʽΪ2O2+8e-+4H2O=8OH-��
�ʴ�Ϊ��CH4-8e-+10OH-=CO32-+7H2O��2O2+8e-+4H2O=8OH-��
��2����A���������У�EԪ�صĵ��ʻ�ԭ����ǿ����EΪNaԪ�أ��õ������ҺΪNaCl��Һ�����ʱ������ӦΪ2Cl-2e-=Cl2����Cl2����ǿ�����ԣ�����ʪ��ĵ⻯�ص�����ֽ����Y�缫�������飬��������ֽ����ɫ��
�ʴ�Ϊ��2Cl-2e-=Cl2���� ��ʪ��ĵ⻯�ص�����ֽ����Y�缫��������ֽ����ɫ��
�ڵ��ʱ��������ӦΪ��2H2O+2e-=2OH-+H2�����������������ʵ���Ϊ
 =0.005mol��
=0.005mol����n��OH-��=2��H2��=2×0.005mol=0.01mol��c��OH-��=
 =0.1mol/L��
=0.1mol/L������c��OH-��?c��H+��=1×10-14����c��H+��=1×10-13mol/L��
PH=-lg��10-13��=13��
�ʴ�Ϊ��13
���������⿼���Ϊ�ۺϣ��漰Ԫ�ص��ƶϣ����ʵ����ʺ���;�Լ��绯ѧ��֪ʶ��ע��缫����ʽ����д������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ




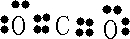
 H++Cl-+HClO
H++Cl-+HClO
