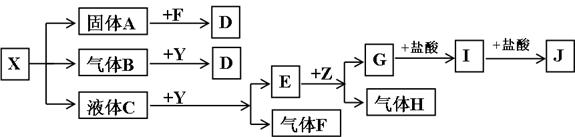
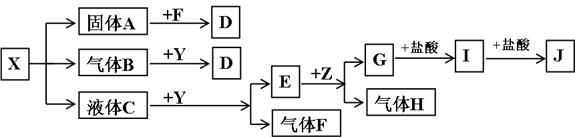
��Ŀ����
��ͼ���������ʾ�Ϊ��ѧ��ѧ�������ʣ�F��H�����嵥�ʣ�Z�ǹ���������ʣ�Y���������������Ӹ�����Ϊ2��1��������X��Y��A��D��E��G����ɫ��ӦΪ��ɫ��I�ǰ�ɫ��������֪��NaAlO2+HCl(����)+H2O![]() NaCl+Al(OH)3��
NaCl+Al(OH)3��
NaAlO2+4HCl(����)![]() NaCl+AlCl3+2H2O
NaCl+AlCl3+2H2O

(1)д��X��E��I�Ļ�ѧʽ��X____________��E____________��I____________��
(2)д��Y�ĵ���ʽ��__________________________________________________��
(3)����Y��˵����ȷ����____________(��ѡ���۷�)��
A.Y�������� B.Y�ǹ������� C.Y��ǿ����� D.Y�Ǽ���������
(4)д��B��Y��Ӧ�Ļ�ѧ����ʽ��____________________________________________��
���������������ϵͼѰ��ͻ�ƿڡ�����֪����ʽ��֪IΪAl(OH)3��ZΪAl���壬HΪH2��EΪNaOH����E������F��֪CΪH2O��YΪNa2O2��FΪO2����A��B��C��֪XΪ��ʽ�Σ���B����Na2O2��Ӧֻ����D��A��O2��Ӧ��D����֪Xֻ��Ϊ���������ʽ�Σ�������Ϊ̼�����ʽ�Σ�����XΪNaHSO3��
�𰸣�(1)NaHSO3 NaOH Al(OH)3
![]()
(3)ABC
(4)SO2+Na2O2![]() Na2SO4
Na2SO4

��ϰ��ϵ�д�
�����Ŀ