��Ŀ����
2000�꣬���Ϸ������缶�ش���ʯ(��TiO2)���Ѻ��ѺϽ�����Ҫ�����������������й㷺����Ҫ��Ӧ�ã�����Ϊ21���͵Ľ�����
(1)��Ԫ�ص�ͬλ���У�![]() ��
��![]() ��
��![]() ��
��![]() ��������˵����ȷ����( )
��������˵����ȷ����( )
A.�ɼ������Ԫ�ص����ԭ������Ϊ48 B.��Ԫ�ص�����ͬλ�ػ�ѧ���ʾ�����ͬ
C.��Ԫ����Ԫ�����ڱ���λ�ڵ������� D.������(26Fe)ͬΪ�ڢ���Ԫ��
(2)TiO2(��������)�Ǹ��İ�ɫ���ϣ����������з�Ӧ�Ƶã�
��һ����FeTiO3+2H2SO4====TiOSO4+FeSO4+2H2O����ʱ�������е�Fe2O3Ҳ��H2SO4������Ӧ���ɼ�����мʹ�仹ԭ��Fe2+,д���˹��̵����ӷ�Ӧ����ʽ��____________________��
�ڶ�����TiOSO4+2H2O![]() TiO2��H2O+H2SO4-Q���ƶ������ѵĹؼ��ǵڶ���ˮ�ⷴӦ��Ϊʹ�ڶ�����Ӧ���еø���ȫ���ɲ�������_______��ʩ��
TiO2��H2O+H2SO4-Q���ƶ������ѵĹؼ��ǵڶ���ˮ�ⷴӦ��Ϊʹ�ڶ�����Ӧ���еø���ȫ���ɲ�������_______��ʩ��
A.���� B.�Ӽ� C.���� D.��ѹ
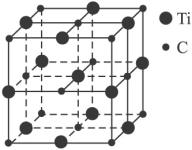
(3)�������һ������ԭ�Ӻ�̼ԭ�ӹ��ɵ���̬�Ŵط��ӣ���ͼ1-26��ʾ�������Ļ�ѧʽΪ_________��
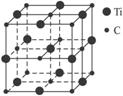
ͼ1-26
��1��C
��2��Fe2O3+6H++Fe====3Fe2++3H2O BC ��3��Ti13C14
����:
��1���ɸ�ͬλ�ص������������Ԫ�صĽ������ԭ������������Ҫ֪������Եİٷֺ�����ͬ��Ԫ�صIJ�ͬͬλ�ػ�ѧ������ͬ����λ�ڵ������ڢ�B�塣
��2����ƽ���ƶ���ԭ����ѡ��B��C��
��3�����ڴ���������̬�Ŵط��ӣ�ֻ���������ɸ÷��ӵ�ԭ�ӵĸ�������Ti13C14��

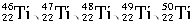 ��������˵����ȷ���� ������
��������˵����ȷ���� ������ TiO2?H2O+H2SO4������ӦΪ���ȷ�Ӧ��
TiO2?H2O+H2SO4������ӦΪ���ȷ�Ӧ��