��Ŀ����
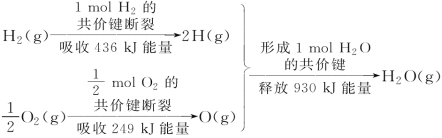
����Ŀ�������仯������NH3����Ρ�N2H4��N2O4������ѧ��ѧ��������ҵ������������ռ����Ҫ��λ��
��1����֪��ӦNO2(g)+CO(g) = NO(g) +CO2(g)�������仯����ͼ��ʾ������˵����ȷ����________��

A��ͼ��A��B�Ĺ���Ϊ���ȹ���
B��1molNO2��1molCO�ļ����ܺʹ���1molNO��1molCO2�ļ����ܺ�
C���÷�ӦΪ������ԭ��Ӧ
D��1molNO2(g)��1molCO(g)������������1molNO(g)��1molCO2(g)��������
��2��N2O4��NO2֮����ڷ�ӦN2O4(g)![]() 2NO2(g)����һ������N2O4��������ܱ������У������ƽ��ת����[��(N2O4)]���¶ȵı仯��ͼ��ʾ��
2NO2(g)����һ������N2O4��������ܱ������У������ƽ��ת����[��(N2O4)]���¶ȵı仯��ͼ��ʾ��

����ͼ�Ʋ�÷�Ӧ�ġ�H___0���>����<����������Ϊ________________��
��ͼ��a���Ӧ�¶��£���֪N2O4����ʼѹǿΪ108 kPa������¶��·�Ӧ��ƽ�ⳣ��Kp=_________kPa����ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�����������
����һ�������£��÷�ӦN2O4��NO2����������������ѹǿ����ڹ�ϵ��( N2O4)=k1p(N2O4)����(NO2)=k2p2(NO2)������k1��k2���뷴Ӧ�¶��йصij�������Ӧ������ѹǿ��ϵ��ͼ��ʾ��һ���¶��£�k1��k2��ƽ�ⳣ��Kp�Ĺ�ϵ��k1=___________������ͼ�ϱ���ĵ��У��ܱ�ʾ��Ӧ�ﵽƽ��״̬�ĵ�Ϊ__________������ĸ���ţ���

��3�����õ�ⷨ����������ˮԭ������ͼ��ʾ�������ĵ缫��ӦʽΪ___________����������Һ�з�����Ӧ�� ________________________����������������Ϊ_______(�ѧʽ)��

���𰸡�C > �¶����ߣ�����N2O4�����ӣ�˵��ƽ�����ƣ��÷�ӦΪ���ȷ�Ӧ����H>0 115.2 Kpk2/2 B����D�� Fe-2e-��Fe2�� Cr2O72��+6Fe2��+14H��=2Cr3�� +6Fe3�� +7H2O H2
��������
���ݻ�ѧƽ�������������𣻸���Ӱ�컯ѧƽ���������ؼ���������ԭ��������������ݵ���ԭ�����������
(1)��ͼ�������֪��A���C���������Ӧ���������������������������ʸ÷�ӦΪ���ȷ�Ӧ��
A.A��B�Ĺ��̻�ѧ�����ѣ�������������A������
B.�÷�Ӧ�Ƿ��ȷ�Ӧ������1molNO2��1molCO�ļ����ܺ�С��1molNO��1molCO2�ļ����ܺͣ���B����
C. ��Ӧǰ��Ԫ�ػ��ϼ۷����仯����Ϊ������ԭ��Ӧ����C��ȷ��
D. �÷�Ӧ�Ƿ��ȷ�Ӧ������1molNO2(g)��1molCO(g)������������1molNO(g)��1molCO2(g)������������D����
��ѡC��
(2) ����ͼ���֪�����¶����ߣ�N2O4��ת��������ƽ�������ƶ�����������Ӧ���������ȷ�Ӧ������H>0���ʴ�ΪΪ��> ���¶����ߣ�����N2O4�����ӣ�˵��ƽ�����ƣ��÷�ӦΪ���ȷ�Ӧ����H>0 ��
��N2O4��ת������0.4����ԭ��N2O4�����ʵ���Ϊxmol��ת����NO2���ʵ���Ϊ0.8xmol��������������ʵ���=��x-0.4x+0.8x��mol=1.4xmol����ͬ�����£������ѹǿ֮�ȵ��������ʵ���֮�ȣ����Է�Ӧ��ѹǿ=![]() =151.2kPa�������������ķ�ѹ=151.2KPa��
=151.2kPa�������������ķ�ѹ=151.2KPa��![]() =64.8kPa�����������ķ�ѹ=151.2KPa��
=64.8kPa�����������ķ�ѹ=151.2KPa��![]() =86.4kPa����ѧƽ�ⳣ��K=
=86.4kPa����ѧƽ�ⳣ��K=![]() =115.2(kPa)��
=115.2(kPa)��
�ۻ�ѧƽ�ⳣ��Kp=[p��NO2��]2��p��N2O4�����ﵽƽ��ʱ�ò�ͬ���ʱ�ʾ�����淴Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ����Ԧԣ�NO2���������ԣ�N2O4������=k2[p��NO2��]2��[k1p��N2O4��]=2��1����ѧƽ�ⳣ��Kp=[p��NO2��]2��p��N2O4����
��k1=![]() k2��Kp������ƽ����������NO2������=2����N2O4��������Ϊƽ��㣬B��D���N2O4��NO2����������֮��Ϊ1��2������B����D��Ϊƽ�����
k2��Kp������ƽ����������NO2������=2����N2O4��������Ϊƽ��㣬B��D���N2O4��NO2����������֮��Ϊ1��2������B����D��Ϊƽ�����
(3)����ͼʾ����������ʧȥ���ӣ���AΪ������BΪ�������缫��ӦʽΪ��
������Fe��2e��=Fe2����������2H����2e��=H2����Ȼ������������Һ����Cr2O72-��6Fe2����14H��=2Cr3����6Fe3����7H2O���ʴ�Ϊ�� Fe-2e-��Fe2���� Cr2O72��+6Fe2��+14H��=2Cr3�� +6Fe3�� +7H2O ��H2��

 �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�