��Ŀ����
����ȷ�ϣ�CO��SO2��NOx���ŷ�����ɴ�����Ⱦ����Ҫԭ��
��1����CO2�������ϳ�CH3OH������Ҫ���壬�ȿ��Խ���������⣬���ɽ����ԴΣ������֪CH3OH������Ҫ���壬�ȿ��Խ���������⣬���ɽ����ԴΣ������֪CH3OH��H2��ȼ���ȷֱ�Ϊ��726.5kJ/mol����285.8kJ/mol��д����ҵ����CO2��H2�ϳ�CH3OH���Ȼ�ѧ����ʽ�� ��
��2���ò����缫��һ��ͨ�������һ��ͨ��CH3OH��l������KOH��Һ�����ȼ�ϵ�أ��为����ӦʽΪ ___����Һ�е��������� �������ƶ���
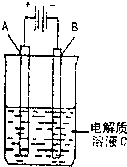
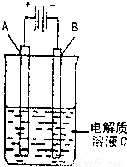
��3����ͼ��һ���绯ѧװ��ʾ��ͼ����CH3OH������ȼ�ϵ������װ�õĵ�Դ��
�����AΪ��ͭ��BΪ��ͭ��CΪCuSO4��Һ����ԭ���Ĺ�ҵ���������� ��
�����A�Dz��缫��B��ʯī�缫��C��AgNO3��Һ��ͨ���
��B������10.8 g����ȼ�ϵ������������____mol�״�����������������λ��Ч���֣�
��4����������1L��0.2 mol/L NaOH��Һ��ͨ��4.48 L����״������
SO2�����Ի�Ϻ���Һ����ı仯�����������Һ��pH<7������Һ��c��SO32����_ c��H2SO3�����>������<������=�������йظ���Һ������Ũ�ȹ�ϵ���ж���ȷ���� ������ĸ��ţ���
A��c��S O32����ʮc��OH����+c��HSO3����=c��Na+��+c��H+�� O32����ʮc��OH����+c��HSO3����=c��Na+��+c��H+�� |
| B��c��H2SO3��+c��HSO3����+c��SO32����=" 0.2" mol/L |
| C��c��H2SO3��+c��H+��=c��SO32����ʮc��OH-��[��Դ:ѧ���ơ���Z��X��X��K] |
| D��c��Na+��>c��H+��>c��HSO3����>c��OH���� |

����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�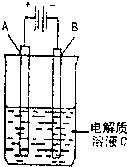 ��2011?̫ԭ��ģ������ȷ�ϣ�CO��SO2��NOx���ŷ�����ɴ�����Ⱦ����Ҫԭ��
��2011?̫ԭ��ģ������ȷ�ϣ�CO��SO2��NOx���ŷ�����ɴ�����Ⱦ����Ҫԭ��