��Ŀ����
2SO2(g)��O2(g)![]() 2SO3(g)����H��a kJ��mol��1����Ӧ���̵������仯��ͼ��ʾ����֪1 mol��SO2(g)��ȫת��Ϊ1 mol��SO3(g)����99 kJ����ش�
2SO3(g)����H��a kJ��mol��1����Ӧ���̵������仯��ͼ��ʾ����֪1 mol��SO2(g)��ȫת��Ϊ1 mol��SO3(g)����99 kJ����ش�
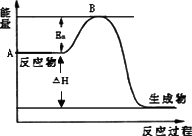
(1)ͼ��A���ʾ________��a��________��
(2)Ea�Ĵ�С�Ը÷�Ӧ�Ħ�H________(��С����ޡ�)Ӱ�죮�÷�Ӧ����V2O5������������V2O5��ʹͼ��B��________(����ߡ��������͡����䡱)��
(3)��֪�������ȼ����Ϊ296 kJ��mol
��1��д����Ӧ���Ȼ�ѧ����ʽ��________�����³�ѹ�£��ɵ������������������Ӧ������3 mol��SO3(g)���ų���������Ϊ________��
�𰸣�

��ϰ��ϵ�д�
�����Ŀ
���ڷ�Ӧ2SO2(g)��O2(g) 2SO3(g)������������Ӧ���ʵĴ�ʩ��
2SO3(g)������������Ӧ���ʵĴ�ʩ��
| A��ͨ�����O2 | B�����������ݻ� | C����ȥ����SO3 | D��������ϵ�¶� |
 2SO3(g)����Ӧ���̵������仯��ͼ��ʾ����֪1 mol SO2(g)����Ϊ1 mol SO3(g)�Ħ�H����99 kJ/mol��
2SO3(g)����Ӧ���̵������仯��ͼ��ʾ����֪1 mol SO2(g)����Ϊ1 mol SO3(g)�Ħ�H����99 kJ/mol��
 2SO3(g)
��H��0��Ӧ��ij�¶��£�SO2��ƽ��ת���ʣ���������ϵ��ѹǿ��p���Ĺ�ϵ����ͼ��ʾ������ͼʾ�ش��������⣺
2SO3(g)
��H��0��Ӧ��ij�¶��£�SO2��ƽ��ת���ʣ���������ϵ��ѹǿ��p���Ĺ�ϵ����ͼ��ʾ������ͼʾ�ش��������⣺
 2SO3(g)����H<0��ij�¶��£���2 mol SO2��1 mol O2����10 L�ܱ������У���Ӧ��ƽ���SO2��ƽ��ת����(��)����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ����ʾ��������˵����ȷ����
2SO3(g)����H<0��ij�¶��£���2 mol SO2��1 mol O2����10 L�ܱ������У���Ӧ��ƽ���SO2��ƽ��ת����(��)����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ����ʾ��������˵����ȷ����