��Ŀ����
�Ǒz�ᣨH3PO3���Ƕ�Ԫ�ᣬ������NaOH��Һ��Ӧ����Na2HPO3��
��1��PCl3ˮ�����ȡ�����PCl3+32O�TH3PO3+______��
��2��H3PO3��Һ�д��ڵ���ƽ�⣺H3PO3?H++H2PO3-��
��ij�¶��£�O.1Omol��L-1 �� H3PO3 ��Һ pH=1.6������Һ�� c��H+��=2.5x 10-2 mol��L-1������¶�����������ƽ���ƽ�ⳣ��K��д��������̣�
��H3PO3�ĵڶ���������Բ��ƣ����������λ��Ч���֣���
�ڸ���H3PO3�����ʿ��Ʋ�Na2HPO3ϡ��Һ��pH______7 �����������=����������
��3�����������ǿ��ԭ�ԣ���ʹ��ˮ��ɫ���÷�Ӧ�Ļ�ѧ����ʽΪ______
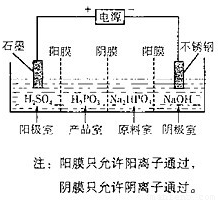
��4�����Na2HPO3��ҺҲ�ɵõ����[�ᣬװ��ʾ��ͼ��ͼ��
�������ĵ缫��ӦʽΪ______
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪ______��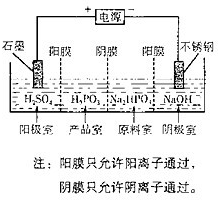
��1��PCl3ˮ�����ȡ�����PCl3+32O�TH3PO3+______��
��2��H3PO3��Һ�д��ڵ���ƽ�⣺H3PO3?H++H2PO3-��
��ij�¶��£�O.1Omol��L-1 �� H3PO3 ��Һ pH=1.6������Һ�� c��H+��=2.5x 10-2 mol��L-1������¶�����������ƽ���ƽ�ⳣ��K��д��������̣�
��H3PO3�ĵڶ���������Բ��ƣ����������λ��Ч���֣���
�ڸ���H3PO3�����ʿ��Ʋ�Na2HPO3ϡ��Һ��pH______7 �����������=����������
��3�����������ǿ��ԭ�ԣ���ʹ��ˮ��ɫ���÷�Ӧ�Ļ�ѧ����ʽΪ______
��4�����Na2HPO3��ҺҲ�ɵõ����[�ᣬװ��ʾ��ͼ��ͼ��
�������ĵ缫��ӦʽΪ______
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪ______��

��1��PCl3ˮ�����ȡ����������ᣬˮ�ⷽ��ʽΪ��PCl3+32O?H3PO3+3HCl���ʴ�Ϊ��HCl��
��2����H3PO3=H++H2PO3-
��ʼŨ�� 0.10 0 0
��ӦŨ�� 2.5��10-2 2.5��10-2 2.5��10-2
ƽ��Ũ��0.10-2.5��10-2 2.5��10-2 2.5��10-2
����ƽ�ⳣ��K=
=
mol/L=8.3��10-3mol/L���ʴ�Ϊ8.3��10-3mol/L��
��H3PO3�����ᣬNa2HPO3��ǿ�������Σ�������ˮ��Һ�ʼ��ԣ���pH��7���ʴ�Ϊ������
��3��������͵ⷢ��������ԭ��Ӧ������������ԭ���������������ᣬ�ⱻ��ԭ��������ᣬ��Ӧ����ʽΪ��H3PO3+I2+H2O=2HI+H3PO4���ʴ�Ϊ��H3PO3+I2+H2O=2HI+H3PO4��
��4���������������ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ2H++2e-=H2�����ʴ�Ϊ��2H++2e-=H2����
�ڲ�Ʒ����HPO32-�������ӽ�����������ᣬ��Ӧ���ӷ���ʽΪ��HPO32-+2H+=H3PO3���ʴ�Ϊ��HPO32-+2H+=H3PO3��
��2����H3PO3=H++H2PO3-
��ʼŨ�� 0.10 0 0
��ӦŨ�� 2.5��10-2 2.5��10-2 2.5��10-2
ƽ��Ũ��0.10-2.5��10-2 2.5��10-2 2.5��10-2
����ƽ�ⳣ��K=
| C(H+)��C(H2PO3-) |
| C(H3PO3) |
| 2.5��10-2��2.5��10-2 |
| 0.10-2.5��10-2 |
��H3PO3�����ᣬNa2HPO3��ǿ�������Σ�������ˮ��Һ�ʼ��ԣ���pH��7���ʴ�Ϊ������
��3��������͵ⷢ��������ԭ��Ӧ������������ԭ���������������ᣬ�ⱻ��ԭ��������ᣬ��Ӧ����ʽΪ��H3PO3+I2+H2O=2HI+H3PO4���ʴ�Ϊ��H3PO3+I2+H2O=2HI+H3PO4��
��4���������������ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ2H++2e-=H2�����ʴ�Ϊ��2H++2e-=H2����
�ڲ�Ʒ����HPO32-�������ӽ�����������ᣬ��Ӧ���ӷ���ʽΪ��HPO32-+2H+=H3PO3���ʴ�Ϊ��HPO32-+2H+=H3PO3��

��ϰ��ϵ�д�
�����Ŀ
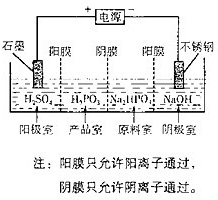 ��2013?����һģ���Ǒz�ᣨH3PO3���Ƕ�Ԫ�ᣬ������NaOH��Һ��Ӧ����Na2HPO3��
��2013?����һģ���Ǒz�ᣨH3PO3���Ƕ�Ԫ�ᣬ������NaOH��Һ��Ӧ����Na2HPO3�� H++H2PO3����
H++H2PO3����