��Ŀ����
�����Ԫ�����ڱ���һ���֣�
��1������Ԫ�آ���⻯��Ļ�ѧʽΪ______�����⻯��Ļ�ԭ�Ա�Ԫ��

���⻯��Ļ�ԭ��______�ȶ���______����ǿ������
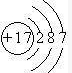
��2��ijԪ�ص�ԭ�ӽṹʾ��ͼΪ��

�������ڱ�λ��______�����⻯��е�ȼ���______��
��3���õ���ʽ��ʾ

����γɻ������γɹ��̣�______��
��4��д������ᡢ����

������������Ӧˮ���ﷴӦ�����ӷ���ʽ������______������

______��
| �� |
I A | II A | III A | IV A | V A | VI A | VII A |
| 2 | �� | �� | �� | �� | �� | ||
| 3 | �� | �� | �� | �� |  |
 |

���⻯��Ļ�ԭ��______�ȶ���______����ǿ������
��2��ijԪ�ص�ԭ�ӽṹʾ��ͼΪ��

�������ڱ�λ��______�����⻯��е�ȼ���______��
��3���õ���ʽ��ʾ

����γɻ������γɹ��̣�______��
��4��д������ᡢ����

������������Ӧˮ���ﷴӦ�����ӷ���ʽ������______������

______��
��1����ΪP�����⻯��Ļ�ѧʽΪPH3��

ΪS���ǽ�����S��P����ԭ��PH3��H2S����̬�⻯��H2S��PH3���ʴ�Ϊ��PH3��ǿ������
��2��K����������Ϊ2����x=2����ԭ�ӽṹ����3�����Ӳ㣬����������Ϊ4����Ԫ�����ڱ��е�λ��Ϊ�������ڵڢ�A�壬��̬�⻯�����Է�������PH3��CH4����PH3�е�ߣ�
�ʴ�Ϊ���������ڵڢ�A�壻�ߣ�
��3��

ΪS����ΪMg���γɵĻ�����Ϊ���ӻ�������γɹ���Ϊ

���ʴ�Ϊ��

��
��4����ΪNa����ΪAl��

ΪCl��NaOH��Al��OH��3��Ӧ�����ӷ�ӦΪOH-+Al��OH��3�TAlO2-+2H2O��HClO4��Al��OH��3��Ӧ�����ӷ�ӦΪ3H++Al��OH��3�TAl3++3H2O��
�ʴ�Ϊ��OH-+Al��OH��3�TAlO2-+2H2O��3H++Al��OH��3�TAl3++3H2O��

ΪS���ǽ�����S��P����ԭ��PH3��H2S����̬�⻯��H2S��PH3���ʴ�Ϊ��PH3��ǿ������
��2��K����������Ϊ2����x=2����ԭ�ӽṹ����3�����Ӳ㣬����������Ϊ4����Ԫ�����ڱ��е�λ��Ϊ�������ڵڢ�A�壬��̬�⻯�����Է�������PH3��CH4����PH3�е�ߣ�
�ʴ�Ϊ���������ڵڢ�A�壻�ߣ�
��3��

ΪS����ΪMg���γɵĻ�����Ϊ���ӻ�������γɹ���Ϊ

���ʴ�Ϊ��

��
��4����ΪNa����ΪAl��

ΪCl��NaOH��Al��OH��3��Ӧ�����ӷ�ӦΪOH-+Al��OH��3�TAlO2-+2H2O��HClO4��Al��OH��3��Ӧ�����ӷ�ӦΪ3H++Al��OH��3�TAl3++3H2O��
�ʴ�Ϊ��OH-+Al��OH��3�TAlO2-+2H2O��3H++Al��OH��3�TAl3++3H2O��

��ϰ��ϵ�д�
 һ����������ϵ�д�
һ����������ϵ�д�
�����Ŀ

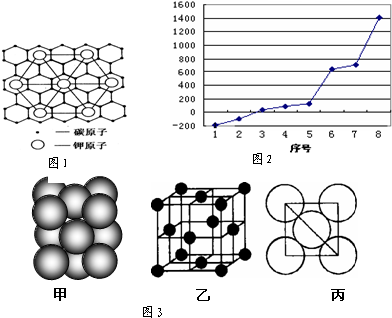
 ���Ŀռ�������Ϊ
���Ŀռ�������Ϊ