��Ŀ����
��2008?����һģ��̼���⡢��3��Ԫ����ɵ��л���A����Է�������Ϊ102���������������Ϊ9.8%����������ԭ�Ӹ���Ϊ����5����
��1��A�ķ���ʽ��
��2��A��2����ͬ�ĺ��������ţ��������������
��3��һ�������£�A��������Ӧ����B��B���ӵĽṹ����Ϊ1��̼ԭ��������2����������2���ṹ��ͬ�Ļ��ţ� ��A�Ľṹ��ʽ��
 ��
��
��A���ܷ����ķ�Ӧ�ǣ���д�����ĸ��
a��ȡ����Ӧb����ȥ��Ӧ c��������Ӧd����ԭ��Ӧ
��4��д��������A������ͬ�����š�������֧����ͬ���칹��Ľṹ��ʽ��
 ��
��
 ��
��
��5��A������һ������ͬ���칹�壬���칹��������������ˮ�⣬����������Է���������ͬ�Ļ��������һ�ֵķ�������2�������˷�Ӧ�Ļ�ѧ����ʽ��
��1��A�ķ���ʽ��
C5H10O2
C5H10O2
�� ��2��A��2����ͬ�ĺ��������ţ��������������
�ǻ���ȩ��
�ǻ���ȩ��
����3��һ�������£�A��������Ӧ����B��B���ӵĽṹ����Ϊ1��̼ԭ��������2����������2���ṹ��ͬ�Ļ��ţ� ��A�Ľṹ��ʽ��


��A���ܷ����ķ�Ӧ�ǣ���д�����ĸ��
b
b
��a��ȡ����Ӧb����ȥ��Ӧ c��������Ӧd����ԭ��Ӧ
��4��д��������A������ͬ�����š�������֧����ͬ���칹��Ľṹ��ʽ��




��5��A������һ������ͬ���칹�壬���칹��������������ˮ�⣬����������Է���������ͬ�Ļ��������һ�ֵķ�������2�������˷�Ӧ�Ļ�ѧ����ʽ��
CH3COOCH��CH3��2+H2O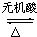 CH3COOH+��CH3��2CH-OH
CH3COOH+��CH3��2CH-OH
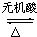 CH3COOH+��CH3��2CH-OH
CH3COOH+��CH3��2CH-OHCH3COOCH��CH3��2+H2O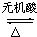 CH3COOH+��CH3��2CH-OH
CH3COOH+��CH3��2CH-OH
��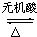 CH3COOH+��CH3��2CH-OH
CH3COOH+��CH3��2CH-OH��������1��������Է��������ͺ������ɼ���һ�������к��е�Hԭ�Ӹ��������ݷ�������ԭ�Ӹ���Ϊ��������5���ɼ���Oԭ�Ӹ�������������Է�����������Cԭ�Ӹ�����������÷���ʽ��
��2�����ݺ��������ŵ����������
��3���ٸ��ݷ���ʽ����л���Ľṹ�жϣ�
�ڸ����л��ﺬ�еĹ������жϿ��ܾ��е����ʣ�
��4������A�Ľṹ��̼���칹��λ���칹��д�ṹ��ʽ��
��5����������ˮ��õ���ʹ�����д����ʽ��
��2�����ݺ��������ŵ����������
��3���ٸ��ݷ���ʽ����л���Ľṹ�жϣ�
�ڸ����л��ﺬ�еĹ������жϿ��ܾ��е����ʣ�
��4������A�Ľṹ��̼���칹��λ���칹��д�ṹ��ʽ��
��5����������ˮ��õ���ʹ�����д����ʽ��
����⣺��1������Է�������Ϊ102���������������Ϊ9.8%��������к��е�N��H��=
=10��
��������ԭ�Ӹ���Ϊ��������5������N��O��=2��
N��C��=
=5��
���Է���ʽΪC5H10O2���ʴ�Ϊ��C5H10O2��
��2������ʽΪC5H10O2���л������Ϊ�ᡢ����������������������ŵĻ�������Ϊ�ʻ����ǻ����ʴ�Ϊ���ǻ����ʻ���
��3����һ�������£�A��һ��������Ӧ����B��B���ӵĽṹ����Ϊһ��̼ԭ�������������������������ṹ��ͬ�Ļ��ţ���BΪ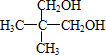 ����ӦΪ
����ӦΪ ��
��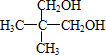 ����AΪ
����AΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��A�к���-CHO���ܷ�����ԭ��Ӧ������-OH���ܷ���ȡ����������Ӧ����������Cԭ����û��Hԭ�ӣ����ܷ�����ȥ��Ӧ���ʴ�Ϊ��b��
��4����A���к���-CHO��-OH����ͬ�����š�������֧����ͬ���칹��Ľṹ��ʽΪ�� ��
�� ��
��
�ʴ�Ϊ ��
�� ������������Ҳ�ɣ���
������������Ҳ�ɣ���
��5��������ˮ��õ���ʹ�������CH3COOCH��CH3��2+H2O CH3COOH+��CH3��2CH-OH���ʴ�Ϊ��CH3COOCH��CH3��2+H2O
CH3COOH+��CH3��2CH-OH���ʴ�Ϊ��CH3COOCH��CH3��2+H2O CH3COOH+��CH3��2CH-OH��
CH3COOH+��CH3��2CH-OH��
| 102��9.8% |
| 1 |
��������ԭ�Ӹ���Ϊ��������5������N��O��=2��
N��C��=
| 102-10-16��2 |
| 12 |
���Է���ʽΪC5H10O2���ʴ�Ϊ��C5H10O2��
��2������ʽΪC5H10O2���л������Ϊ�ᡢ����������������������ŵĻ�������Ϊ�ʻ����ǻ����ʴ�Ϊ���ǻ����ʻ���
��3����һ�������£�A��һ��������Ӧ����B��B���ӵĽṹ����Ϊһ��̼ԭ�������������������������ṹ��ͬ�Ļ��ţ���BΪ
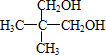 ����ӦΪ
����ӦΪ ��
��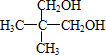 ����AΪ
����AΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����A�к���-CHO���ܷ�����ԭ��Ӧ������-OH���ܷ���ȡ����������Ӧ����������Cԭ����û��Hԭ�ӣ����ܷ�����ȥ��Ӧ���ʴ�Ϊ��b��
��4����A���к���-CHO��-OH����ͬ�����š�������֧����ͬ���칹��Ľṹ��ʽΪ��
 ��
�� ��
���ʴ�Ϊ
 ��
�� ������������Ҳ�ɣ���
������������Ҳ�ɣ�����5��������ˮ��õ���ʹ�������CH3COOCH��CH3��2+H2O
 CH3COOH+��CH3��2CH-OH���ʴ�Ϊ��CH3COOCH��CH3��2+H2O
CH3COOH+��CH3��2CH-OH���ʴ�Ϊ��CH3COOCH��CH3��2+H2O CH3COOH+��CH3��2CH-OH��
CH3COOH+��CH3��2CH-OH�����������⿼���л������ʽ��ȷ�����Լ��л�������ŵĽṹ�����ʣ���Ŀ�Ѷ��еȣ������״���Ϊ��3����ע��ṹ��ʽ���ƶϣ�

��ϰ��ϵ�д�
 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�
�����Ŀ



 +O2
+O2 +2H2O
+2H2O