��Ŀ����
����������мг���������ʡ�ԣ��������ͼ�ش����⣺

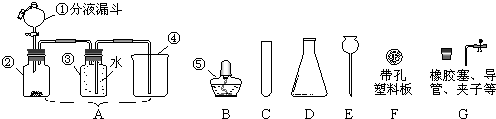
��1��ͼ�������ܢݵ����ƣ���______����______��
��2��ʵ������ȡ������ijͬѧ�����ͼA��װ�ã������������ռ����������У���������ʢ�ŵ��Լ�ӦΪ______���˷����������Ļ�ѧ����ʽΪ______��
��3��ʵ������ȡ������̼����B-G��ѡ����������װ����װ�ã�Ҫ���ܷ�����Ʒ�Ӧ�ķ�����ֹͣ�������ȷѡ��Ϊ������ĸ��______����Ӧ�Ļ�ѧ����ʽΪ______��
��4����ͼ��������ԭ����ͭ����ʵ��װ��ͼ������ͼA��ȣ���ҩˮƿ�������൱����������������ţ�______��


��1��ͼ�������ܢݵ����ƣ���______����______��
��2��ʵ������ȡ������ijͬѧ�����ͼA��װ�ã������������ռ����������У���������ʢ�ŵ��Լ�ӦΪ______���˷����������Ļ�ѧ����ʽΪ______��
��3��ʵ������ȡ������̼����B-G��ѡ����������װ����װ�ã�Ҫ���ܷ�����Ʒ�Ӧ�ķ�����ֹͣ�������ȷѡ��Ϊ������ĸ��______����Ӧ�Ļ�ѧ����ʽΪ______��
��4����ͼ��������ԭ����ͭ����ʵ��װ��ͼ������ͼA��ȣ���ҩˮƿ�������൱����������������ţ�______��

��1��������Ҫ����дʵ��װ���е��������ƣ����ձ����ݾƾ��ƣ�
�ʴ�Ϊ���ձ����ƾ��ƣ�
��2���˷�Ӧװ���ʺϹ�Һ��Ӧ����Ҫ���ȵķ�Ӧ�����ѡ���ʺϷ�Ӧװ�õ�ҩƷ�ǣ�H2O2�����������Ļ�ѧ����ʽΪ��
2H2O2
.2H2O+O2����
�ʴ�Ϊ��H2O2�� 2H2O2
.2H2O+O2����
��3��ʵ���ҳ��ô���ʯ��ϡ������ȡ������̼����Ҫ���ܷ�����Ʒ�Ӧ�ķ�����ֹͣʱ����Ҫ��Ӧ��֮��˴��ܹ����룬���Ծ��õ�F�����Դ�ʱ�Ͳ��˲�����ƿ��Ȼ��ѡ��CEFG������������װ���ﵽҪ��
��Ӧ�ķ���ʽΪ��CEFG��CaCO3+2HCl=CaCl2+H2O+CO2����
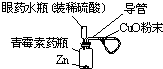
��4�����Ƚ���װ��֪����ҩˮƿ�������൱��������Һ©�������٣�
��ѡ �٣�
�ʴ�Ϊ���ձ����ƾ��ƣ�
��2���˷�Ӧװ���ʺϹ�Һ��Ӧ����Ҫ���ȵķ�Ӧ�����ѡ���ʺϷ�Ӧװ�õ�ҩƷ�ǣ�H2O2�����������Ļ�ѧ����ʽΪ��
2H2O2
| ||
�ʴ�Ϊ��H2O2�� 2H2O2
| ||
��3��ʵ���ҳ��ô���ʯ��ϡ������ȡ������̼����Ҫ���ܷ�����Ʒ�Ӧ�ķ�����ֹͣʱ����Ҫ��Ӧ��֮��˴��ܹ����룬���Ծ��õ�F�����Դ�ʱ�Ͳ��˲�����ƿ��Ȼ��ѡ��CEFG������������װ���ﵽҪ��
��Ӧ�ķ���ʽΪ��CEFG��CaCO3+2HCl=CaCl2+H2O+CO2����
��4�����Ƚ���װ��֪����ҩˮƿ�������൱��������Һ©�������٣�
��ѡ �٣�

��ϰ��ϵ�д�
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
�����Ŀ