��Ŀ����
19����ͼ��ʾ�й����ʣ����ɶ�����Ԫ���γɣ�֮���ת����ϵ������AΪ�����Ľ������ʣ�BΪ�ǽ������ʣ�һ���Ǻ�ɫ��ĩ����C�dz�������ɫ��ζҺ�壬D�ǵ���ɫ�Ĺ��廯�������Ӧ����ͼ����ʡ�ԣ�
��1��A��B��C��D���������ʷֱ�ΪAl��C��H2O��Na2O2���ѧʽ����
��2����Ӧ���е�C��D���������÷�Ӧ�Ļ�ѧ����ʽ��2H2O+2Na2O2=4NaOH+O2����2Al+2NaOH+2H2O=2NaAlO2+3H2����
��3����Ӧ�۲�����K�Ļ�ѧʽΪNa2CO3��
��4����Ӧ�ܵ����ӷ���ʽΪ2AlO2-+CO2+3H2O=2Al��OH��3��+CO32-��
���� ת����ϵ�и����ʾ��ɶ�����Ԫ����ɣ�AΪ�����Ľ������ʣ���AΪAl��BΪ�ǽ������ʣ�һ���Ǻ�ɫ��ĩ����BΪ̼��C�dz�������ɫ��ζҺ�壬CΪH2O��D�ǵ���ɫ�Ĺ��廯�����DΪNa2O2���ɷ�Ӧ�ٿ�֪��ΪNaAlO2��E��F�ֱ�ΪO2��H2�е�һ�֣�B����F��Ӧ����G��H����G����������Ʒ�Ӧ����F��K������֪FΪO2��EΪH2��GΪCO2��HΪCO��KΪNa2CO3���ɷ�Ӧ�ܿ�֪LΪAl��OH��3����Һ��ΪNa2CO3��Һ���ݴ˽��
��� �⣺ת����ϵ�и����ʾ��ɶ�����Ԫ����ɣ�AΪ�����Ľ������ʣ���AΪAl��BΪ�ǽ������ʣ�һ���Ǻ�ɫ��ĩ����BΪ̼��C�dz�������ɫ��ζҺ�壬CΪH2O��D�ǵ���ɫ�Ĺ��廯�����DΪNa2O2���ɷ�Ӧ�ٿ�֪��ΪNaAlO2��E��F�ֱ�ΪO2��H2�е�һ�֣�B����F��Ӧ����G��H����G����������Ʒ�Ӧ����F��K������֪FΪO2��EΪH2��GΪCO2��HΪCO��KΪNa2CO3���ɷ�Ӧ�ܿ�֪LΪAl��OH��3����Һ��ΪNa2CO3��Һ��
��1����������������֪AΪAl��BΪC��CΪH2O��DΪNa2O2��
�ʴ�Ϊ��Al��C��H2O��Na2O2��
��2����Ӧ���е�C��D�����������ɵ�����������Һ�ܺ�Al��ȫ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽ�ǣ�2H2O+2Na2O2=4NaOH+O2����2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��2H2O+2Na2O2=4NaOH+O2����2Al+2NaOH+2H2O=2NaAlO2+3H2����
��3��������������֪��KΪNa2CO3���ʴ�Ϊ��Na2CO3��
��4����Ӧ�ܵ����ӷ���ʽΪ��2AlO2-+CO2+3H2O=2Al��OH��3��+CO32-���ʴ�Ϊ��2AlO2-+CO2+3H2O=2Al��OH��3��+CO32-��
���� ���⿼��������ƶϣ��Ѷ��еȣ�ע��������ת����ϵ�����ʾ�Ϊ������Ԫ����ɣ������Ŀ��Ϣ��AΪ�������������ʵ���ɫ��ת����ϵ�����ⷴӦ�����ƶϣ���Ҫѧ����������Ԫ�ػ�����֪ʶ��

| A�� | ��״���£�0.1NA��ˮ������ռ�����Լ��2.24 L | |
| B�� | ���³�ѹ�£�11.2 L CO2�к���11NA������ | |
| C�� | CH4��Ħ��������NA��������ӵ�������� | |
| D�� | �����£�64gSO2�����к��е�ԭ����Ϊ3NA |
| A�� | CO2��CO������ȼ | |
| B�� | ����أ��Ȼ��ƣ���Ũ��Һ���½ᾧ | |
| C�� | MgSO4��MgCl2�������ɡ����� | |
| D�� | ���ᣨ��ȩ����������������ͭ��Һ���ȣ����� |
 ��NaOH��Һ��ͨ��CO2�����õ���ҺM����CO2ͨ�������ͬ����ҺM�����Ҳ��ͬ������M����μ������ᣬ�������������V��CO2���������������V��HCl���Ĺ�ϵ��ͼ�������з�����ȷ���ǣ�����CO2�ܽ⣩��������
��NaOH��Һ��ͨ��CO2�����õ���ҺM����CO2ͨ�������ͬ����ҺM�����Ҳ��ͬ������M����μ������ᣬ�������������V��CO2���������������V��HCl���Ĺ�ϵ��ͼ�������з�����ȷ���ǣ�����CO2�ܽ⣩��������| A�� | ��OB=0������ҺMΪNa2CO3��Һ | |
| B�� | ��OB=BC�����γ���ҺM��������Ӧ�����ӷ���ʽΪOH-+CO2��HCO3- | |
| C�� | ����ҺM��c��NaHCO3��=2c��Na2CO3������3OB=BC | |
| D�� | ����ҺM�д������ڵ�������ΪCO32-��HCO3-����OB��BC |
| A�� | ʹ��̪��Һ������Һ��Na+��Cl-��SO42-��Fe3+ | |
| B�� | ǿ������Һ�У�Na+��K+��NO3-��SiO32- | |
| C�� | ����Al��Ӧ�ų���������Һ�У�Na+��Ca2+��Cl-��HCO3- | |
| D�� | ʹʯ���������Һ�У�Ba2+��Na+��AlO2-��Cl- |
| A�� | SiO2��CO2 �������������������NaOH��Һ��Ӧ | |
| B�� | Na2O��Na2O2���Ԫ����ͬ����CO2��Ӧ�IJ�����ͬ | |
| C�� | SO2��NO��CO2���Ǵ�����Ⱦ��ڿ����ж����ȶ����� | |
| D�� | HCl��HNO3����ǿ�ᣬ��FeO�ķ�Ӧ�����ڸ��ֽⷴӦ |
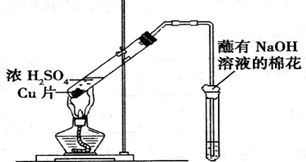
| �Թ����Լ� | �Թ������� | �� �� | |
| A | �����ữ��BaCl2��Һ | ���ɰ�ɫ���� | ��ɫ����ΪBaSO3 |
| B | Ʒ����Һ | ��Һ��ɫ | SO2����Ư���� |
| C | ��ɫʯ����Һ | ��Һ��� | SO2ˮ��Һ������ |
| D | ����KMnO4��Һ | ��ɫ��ȥ | SO2���л�ԭ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | һ����Cl2 | B�� | һ����SO2 ��NO | ||
| C�� | ������NO2 | D�� | һ����SO2��������NO |
 ��
��