��Ŀ����
.(12��)ij���Ͻ�Ӳ�����к���þ��ͭ���裬Ϊ�˲ⶨ�úϽ������ĺ�����ijͬѧ����������飺
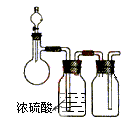
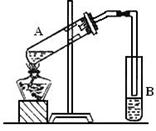
(1)ȡ��Ʒ ag ����ȡʱʹ�õ���������Ϊ ��
(2)����Ʒ����������ϡ�����У����ˣ������к��� �����ܽ����ʱʹ�õIJ�����
���� ��
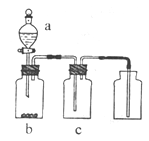
(3)����Һ�м��������NaOH��Һ�����ˣ�д���ò��������йص����ӷ���ʽ ��
(4)�ڵ�( 3 )������Һ��ͨ������CO2�����ˡ��йط�Ӧ�����ӷ���ʽ�� ��
(5)���裨4�����˺������������ˮϴ�����κ�ɲ��������������ټ���Ϊֹ����ȴ�����������Ϊbg����ԭ��Ʒ���������������� ��
(6)�����裨3���м����������Ƶ������㣬��ʵ����ƫ ����ߡ������͡���Ӱ�족����ͬ���������裨5��������û��ϴ�ӣ���ʵ����ƫ ��������ȼ�ղ���֣���ʵ����ƫ ��
(1)ȡ��Ʒ ag ����ȡʱʹ�õ���������Ϊ ��
(2)����Ʒ����������ϡ�����У����ˣ������к��� �����ܽ����ʱʹ�õIJ�����
���� ��
(3)����Һ�м��������NaOH��Һ�����ˣ�д���ò��������йص����ӷ���ʽ ��
(4)�ڵ�( 3 )������Һ��ͨ������CO2�����ˡ��йط�Ӧ�����ӷ���ʽ�� ��
(5)���裨4�����˺������������ˮϴ�����κ�ɲ��������������ټ���Ϊֹ����ȴ�����������Ϊbg����ԭ��Ʒ���������������� ��
(6)�����裨3���м����������Ƶ������㣬��ʵ����ƫ ����ߡ������͡���Ӱ�족����ͬ���������裨5��������û��ϴ�ӣ���ʵ����ƫ ��������ȼ�ղ���֣���ʵ����ƫ ��
(1)������ƽ (2)�衢ͭ���ձ���©����������
(3) Al3+ + 4OH�� = [Al(OH)4]����Mg2+ + 2OH�� = Mg(OH)2��
(4) [Al(OH)4]��+ CO2 = Al(OH)3��+ HCO3�� (5) 9b/17a (6) �� ���� ����
(3) Al3+ + 4OH�� = [Al(OH)4]����Mg2+ + 2OH�� = Mg(OH)2��
(4) [Al(OH)4]��+ CO2 = Al(OH)3��+ HCO3�� (5) 9b/17a (6) �� ���� ����
��1����������ҩƷʹ��������ƽ��
��2��ͭ����������Ӧ������������ͭ�衣�ܽ����ʱʹ�õIJ����������ձ���©������������
��3������ǿ������������þ���������������������������������������������ǿ���С��йصķ���ʽΪAl3+ + 4OH�� = [Al(OH)4]����Mg2+ + 2OH�� = Mg(OH)2����
��4����Һ�к���ƫ�����Σ�ͨ��CO2����������������������ʽΪ[Al(OH)4]��+ CO2 = Al(OH)3��+ HCO3����
��5���������������������յõ���������������������������Ϊ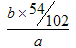 ��9b/17a��
��9b/17a��
��6�������裨3���м����������Ƶ������㣬����Һ��ƫ�����ε�����ƫ�ͣ������ƫ�͡������裨5��������û��ϴ�ӣ���ʵ����ƫ������������������ƫ���ƫ�ߡ�������ȼ�ղ���֣�����������ƫ�ߣ����ƫ�ߡ�
��2��ͭ����������Ӧ������������ͭ�衣�ܽ����ʱʹ�õIJ����������ձ���©������������
��3������ǿ������������þ���������������������������������������������ǿ���С��йصķ���ʽΪAl3+ + 4OH�� = [Al(OH)4]����Mg2+ + 2OH�� = Mg(OH)2����
��4����Һ�к���ƫ�����Σ�ͨ��CO2����������������������ʽΪ[Al(OH)4]��+ CO2 = Al(OH)3��+ HCO3����
��5���������������������յõ���������������������������Ϊ
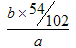 ��9b/17a��
��9b/17a����6�������裨3���м����������Ƶ������㣬����Һ��ƫ�����ε�����ƫ�ͣ������ƫ�͡������裨5��������û��ϴ�ӣ���ʵ����ƫ������������������ƫ���ƫ�ߡ�������ȼ�ղ���֣�����������ƫ�ߣ����ƫ�ߡ�

��ϰ��ϵ�д�
�����Ŀ