��Ŀ����
ij��ѧ��ȤС�����ô�����ͭ(������ͭ�ۡ����������������������)��ȡ
��ˮ�Ȼ�ͭ�����Ʊ��������£�
ʵ����������õ�����X��Y��pH���Ʋ����±�ȷ����
��I
| ���� | ��ʼ����ʱpH | ��ȫ����ʱpH |
| Fe(OH)3 | 2��7 | 3��7 |
| Fe(OH)2 | 7��6 | 9��6 |
| Cu(OH)2 | 5��2 | 6��4 |
����
| ������ | ����pH������ | ||
| A | ˫��ˮ | D | ��ˮ |
| B | ������� | E | ��ʽ̼��ͭ |
| C | ��ˮ | F | ����ͭ |
����д���пհ�
(1)������ijɷ�(��ѧʽ)�� ��
(2)����ڼ�����Լ�X��ѡ�ñ����е� (�����)���������� ��
(3)����ۼ�����Լ�Y��ѡ�ñ����е� (�����)������pH��5��Ŀ���� ��
(4)�����Ҫ�õ���ˮCuCl2��Ӧ���Ƶ������� ��
(5)���������������ȫ����Ӧ�����ӷ���ʽ��
��
��1��Fe��OH��3
��2��AC ��Fe2+����ΪFe3+
��3��EF ������Һ��pH��ʹFe3+��ȫת��Ϊ��������Cu2+��������
��4����HCl�������н�����ˮ
��5��CuO+ 2H+=
Fe2O3+6H+=
Fe3++Cu=
����:��

 ��У����ϵ�д�
��У����ϵ�д�
��1 ���ʳ���ʱ��pH
�� �� | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 |
��ʼ����ʱ��pH | 2.7 | 7.6 | 5.2 |
��ȫ������pH | 3.7 | 9.6 | 6.4 |
��2 ��ѡ�Լ�
�� �� | A | B | C | D | E |
��ѧʽ | NaOH | CuO | H2O2 | ϡHNO3 | KMnO4 |
�ش��������⣺
��1��X_________��Y_________������д��ѡ�Լ��е���ţ�
��2���������Ļ�ѧʽ_____��֤��������г����Ƿ���ȫ�IJ���������____________��
��3���ڢݲ�������У�����װ����ͼ��ʾ��

�������������ҵķ���װ�ýӿڵ�����˳��Ϊ_________��
Dװ�õ�������______________________������______________________��
ij��ѧ��ȤС�����ô�����ͭ(������ͭ�ۡ����������������������)��ȡ
��ˮ�Ȼ�ͭ�����Ʊ��������£�
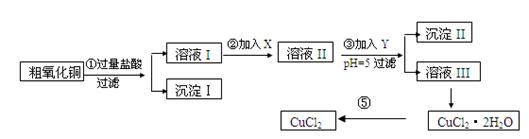
ʵ����������õ�����X��Y��pH���Ʋ����±�ȷ����
��I
| ���� | ��ʼ����ʱpH | ��ȫ����ʱpH |
| Fe(OH)3 | 2��7 | 3��7 |
| Fe(OH)2 | 7��6 | 9��6 |
| Cu(OH)2 | 5��2 | 6��4 |
����
| ������ | ����pH������ | ||
| A | ˫��ˮ | D | ��ˮ |
| B | ������� | E | ��ʽ̼��ͭ |
| C | ��ˮ | F | ����ͭ |
����д���пհ�
(1)������ijɷ�(��ѧʽ)�� ��
(2)����ڼ�����Լ�X��ѡ�ñ����е� (�����)���������� ��
(3)����ۼ�����Լ�Y��ѡ�ñ����е� (�����)������pH��5��Ŀ���� ��
(4)�����Ҫ�õ���ˮCuCl2��Ӧ���Ƶ������� ��
(5)���������������ȫ����Ӧ�����ӷ���ʽ��
��
ij��ѧ��ȤС�����ô�����ͭ(������ͭ�ۡ����������������������)��ȡ
��ˮ�Ȼ�ͭ�����Ʊ��������£�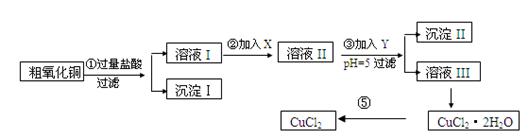
ʵ����������õ�����X��Y��pH���Ʋ����±�ȷ����
��I
| ���� | ��ʼ����ʱpH | ��ȫ����ʱpH |
| Fe(OH)3 | 2��7 | 3��7 |
| Fe(OH)2 | 7��6 | 9��6 |
| Cu(OH)2 | 5��2 | 6��4 |
| ������ | ����pH������ | ||
| A | ˫��ˮ | D | ��ˮ |
| B | ������� | E | ��ʽ̼��ͭ |
| C | ��ˮ | F | ����ͭ |
(1)������ijɷ�(��ѧʽ)�� ��
(2)����ڼ�����Լ�X��ѡ�ñ����е� (�����)���������� ��
(3)����ۼ�����Լ�Y��ѡ�ñ����е� (�����)������pH��5��Ŀ���� ��
(4)�����Ҫ�õ���ˮCuCl2��Ӧ���Ƶ������� ��
(5)���������������ȫ����Ӧ�����ӷ���ʽ��
��
ij��ѧ��ȤС�����ô�����ͭ(������ͭ�ۡ����������������������)��ȡ
��ˮ�Ȼ�ͭ�����Ʊ��������£�
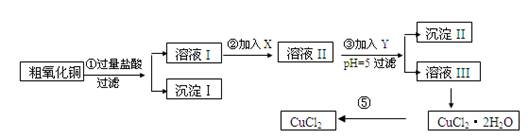
ʵ����������õ�����X��Y��pH���Ʋ����±�ȷ����
��I
|
���� |
��ʼ����ʱpH |
��ȫ����ʱpH[��Դ:Zxxk.Com] |
|
Fe(OH)3 |
2��7 |
3��7 |
|
Fe(OH)2 |
7��6 |
9��6 |
|
Cu(OH)2 |
5��2 |
6��4 |
����
|
������ |
����pH������ |
||
|
A |
˫��ˮ |
D |
��ˮ |
|
B |
������� |
E |
��ʽ̼��ͭ |
|
C |
��ˮ |
F |
����ͭ |
����д���пհ�
(1)������ijɷ�(��ѧʽ)�� ��
(2)����ڼ�����Լ�X��ѡ�ñ����е� (�����)���������� ��
(3)����ۼ�����Լ�Y��ѡ�ñ����е� (�����)������pH��5��Ŀ���� ��
(4)�����Ҫ�õ���ˮCuCl2��Ӧ���Ƶ������� ��
(5)���������������ȫ����Ӧ�����ӷ���ʽ��
��