��Ŀ����
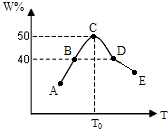 ��5�����ʵ�����Ϊ2.0mol��X��Y��ϣ��ֱ�����¶Ȳ�ͬ���ݻ���ͬ����Ϊ2L���ĺ��������з�����Ӧ2X��g��+Y��g��?3W��g����������ͬʱ��10sʱ�����������W������ٷֺ�����W%�����¶ȣ�T���仯�Ĺ�ϵ��ͼ��
��5�����ʵ�����Ϊ2.0mol��X��Y��ϣ��ֱ�����¶Ȳ�ͬ���ݻ���ͬ����Ϊ2L���ĺ��������з�����Ӧ2X��g��+Y��g��?3W��g����������ͬʱ��10sʱ�����������W������ٷֺ�����W%�����¶ȣ�T���仯�Ĺ�ϵ��ͼ����1��ǰ10sB�����е�ƽ����Ӧ����v��Y��=
2.7��10-2mol/L?s
2.7��10-2mol/L?s
���˷�Ӧ������ӦΪ��
��
�ȷ�Ӧ����2��10sʱA������v��
��
��
v������=��������ͬ����B��D������������Ӧ���ʹ�ϵvB��
��
vD����3����10sʱC��������W���������Ϊ50.0%����X��ת����Ϊ
66.7%
66.7%
����4����10sʱ����E�������¶���������䣬��E��������ͨ��0.6molW��Ҫ�����´ﵽƽ��ʱ������и���ֵİٷֺ������䣬���ʱ����ͬʱͨ��
0.2
0.2
mol��Ӧ��Y����������1������������������Ե����ʵ�����Ȼ������v=
����v��Y���������¶ȵļ������ߣ�W�����ʵ���������С��˵���¶�����ƽ�����ƣ�
��2�������¶ȵ����ߣ���Ӧ������Ӧ������У����¶ȴﵽT0ʱ��W�����ʵ����������˵���ﵽ��Ӧ������ȣ���ƽ��״̬��10sʱA��B������δ����ƽ�⣬C��D��E��������ƽ�⣻�����¶�Խ�ߣ���ѧ��Ӧ����Խ�죻
��3������������������Ե����ʵ�����Ȼ�����ת���ʵĹ�ʽ�����㣻
��4�����ݺ��¡������£�ǰ�������������Ŀ��淴Ӧ�����ɵ�Чƽ�⣬����ѧ������ת����һ�������Ӧ���ʵ����ʵ���֮�Ȳ��伴�ɣ�
| ��c |
| ��t |
��2�������¶ȵ����ߣ���Ӧ������Ӧ������У����¶ȴﵽT0ʱ��W�����ʵ����������˵���ﵽ��Ӧ������ȣ���ƽ��״̬��10sʱA��B������δ����ƽ�⣬C��D��E��������ƽ�⣻�����¶�Խ�ߣ���ѧ��Ӧ����Խ�죻
��3������������������Ե����ʵ�����Ȼ�����ת���ʵĹ�ʽ�����㣻
��4�����ݺ��¡������£�ǰ�������������Ŀ��淴Ӧ�����ɵ�Чƽ�⣬����ѧ������ת����һ�������Ӧ���ʵ����ʵ���֮�Ȳ��伴�ɣ�
����⣺��1��2X��g��+Y��g��?3W��g��
��ʼ��mol�� 2 2 0
��Ӧ��mol�� 2a a 3a
10sĩ��mol��2-2a 2-a 3a
10sĩW������ٷֺ���Ϊ
��100%=40%����ã�a=0.53��
ǰ10sB�����е�ƽ����Ӧ����v��Y��=
=
=2.7��10-2mol/L?s��
�����¶ȵļ������ߣ�W�����ʵ���������С��˵���¶�����ƽ�����ƣ�������ӦΪ���ȷ�Ӧ��
�ʴ�Ϊ��2.7��10-2mol/L?s���ţ�
��2�������¶ȵ����ߣ���Ӧ������Ӧ������У����¶ȴﵽT0ʱ��W�����ʵ����������˵���ﵽ��Ӧ������ȣ���ƽ��״̬��10sʱA��B������δ����ƽ�⣬10sʱA������v����v����
�����¶�Խ�ߣ���ѧ��Ӧ����Խ������B��D������������Ӧ���ʹ�ϵvB��vD
�ʴ�Ϊ����������
��3��2X��g��+Y��g��?3W��g��
��ʼ��mol�� 2 2 0
��Ӧ��mol�� 2a a 3a
10sĩ��mol��2-2a 2-a 3a
10sĩW������ٷֺ���Ϊ
��100%=50%����ã�a=0.667��
��X��ת����Ϊ
��100%=66.7%��
�ʴ�Ϊ��66.7%��
��4��2X��g��+Y��g��?3W��g����
0.4mol 0.2mol 0.6mol
����
=
����ã�n��Y��=0.2mol��
�ʴ�Ϊ��66.7%��0.2��
��ʼ��mol�� 2 2 0
��Ӧ��mol�� 2a a 3a
10sĩ��mol��2-2a 2-a 3a
10sĩW������ٷֺ���Ϊ
| 3a |
| 4 |
ǰ10sB�����е�ƽ����Ӧ����v��Y��=
| ||
| ��t |
| ||
| 10s |
�����¶ȵļ������ߣ�W�����ʵ���������С��˵���¶�����ƽ�����ƣ�������ӦΪ���ȷ�Ӧ��
�ʴ�Ϊ��2.7��10-2mol/L?s���ţ�
��2�������¶ȵ����ߣ���Ӧ������Ӧ������У����¶ȴﵽT0ʱ��W�����ʵ����������˵���ﵽ��Ӧ������ȣ���ƽ��״̬��10sʱA��B������δ����ƽ�⣬10sʱA������v����v����
�����¶�Խ�ߣ���ѧ��Ӧ����Խ������B��D������������Ӧ���ʹ�ϵvB��vD
�ʴ�Ϊ����������
��3��2X��g��+Y��g��?3W��g��
��ʼ��mol�� 2 2 0
��Ӧ��mol�� 2a a 3a
10sĩ��mol��2-2a 2-a 3a
10sĩW������ٷֺ���Ϊ
| 3a |
| 4 |
��X��ת����Ϊ
| 2a |
| 2 |
�ʴ�Ϊ��66.7%��
��4��2X��g��+Y��g��?3W��g����
0.4mol 0.2mol 0.6mol
����
| 0.4 |
| 0.2+n(Y) |
| 2 |
| 2 |
�ʴ�Ϊ��66.7%��0.2��
������������Ҫ�����˻�ѧ��Ӧ���ʵļ��㣬��������Ի�ѧ��Ӧ���ʡ���ѧƽ���Ӱ�졢��Чƽ���֪ʶ���Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
��������淋Ļ�ѧʽΪ��NH4��2Fe��SO4��2?6H2O����Ʒ��ΪĦ���Σ������������������������������泥�һ�������������ڿ������ױ����������γ�Ħ���κ�ͱȽ��ȶ��ˣ������ε��ܽ�ȣ���λΪg/100gˮ�����±�
| �¶�/�� | 10 | 20 | 30 | 40 | 50 | 70 |
| ��NH4��2SO4 | 73.0 | 75.4 | 78.0 | 81.0 | 84.5 | 91.9 |
| FeSO4?7H2O | 40.0 | 48.0 | 60.0 | 73.3 | - | - |
| ��NH4��2Fe��SO4��2?6H2O | 18.1 | 21.2 | 24.5 | 27.9 | 31.3 | 38.5 |
��1�����ʵ�鲽���е���գ�
�ٳ�ȡ4g��м������С�ձ�������15mL10%Na2CO3��Һ��С�����10min�Գ�ȥ______��������Һ��������ˮ������ϴ�ɾ���������ã�
�ڽ������õ���м����С�ձ�������15mL 3mol/L H2SO4��Һ��ˮԡ��������������������Ϊֹ�����ȹ���ȥʣ����м������������ˮϴ���ձ�����ֽ��������Һ��ϴ��Һһ��ת�Ƶ��������У�������������Һ������Ϊ______�������ƣ�������ˮ�⣩�������ʵ���ԼΪ______mol��
�ۼ���______g����淋��������У��������ȡ�Ũ����������ֽᾧĤΪֹ����ȴ������������茶��壮
��2���ش��������⣺
������������Һ�ڿ������ױ��������ʣ�����ʱӦע�⣺a______��b______��
�ڼ��ȡ�Ũ����Һʱ����Ũ�����ɵ�������______��
����������������淋Ļ����Һ����Ũ�����ܵõ���������茶��壬��ԭ����______��
�ܳ�ȡ����������Ϊ1.96g�ĸ���������泥��Ƴ���Һ����δ֪Ũ�ȵ�KMnO4������Һ���еζ���
�� �ζ�ʱ����KMnO4������Һװ��______����ʽ���ʽ���ζ��ܣ�����ʱ��______������ƿ��
�� ��֪MnO4-����ԭΪMn2+����д���õζ������е����ӷ���ʽ��______��
�� �жϸ÷�Ӧ����ζ��յ������Ϊ______��
���� ���赽��ζ��յ�ʱ����ȥV mL KMnO4������Һ�����KMnO4������Һ��Ũ��Ϊ______mol/L��
 �к���һ����Һ������������Ϊ
�к���һ����Һ������������Ϊ ����
���� ��
��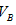 �Ĺ�ϵ��__________ ��
�Ĺ�ϵ��__________ �� ��
��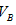 �Ĺ�ϵ��__________ ��
�Ĺ�ϵ��__________ ��