ΧβΡΩΡΎ»ί
Θ®12Ζ÷Θ©Θ°œ¬±μ±ξ≥ωΒΡ «‘ΣΥΊ÷ήΤΎ±μΒΡ“Μ≤ΩΖ÷‘ΣΥΊΘ§ΜΊ¥πœ¬Ν–Έ Χβ
|
1 |
a |
|
|
||||||
|
2 |
|
|
|
|
b |
c |
d |
e |
|
|
3 |
f |
|
g |
h |
|
|
i |
j |
Θ®1Θ©±μ÷–Ν–≥ωΒΡ10÷÷‘ΣΥΊ÷–Θ§Μ·―ß–‘÷ Ήν≤ΜΜνΤΟΒΡ «___________Θ®Χν‘ΣΥΊΟϊ≥ΤΘ§œ¬Ά§Θ©Θ§
Ζ«Ϋπ τ–‘Ήν«ΩΒΡ «___________Θ§‘≠Ή”ΑκΨΕΉν–ΓΒΡ «___________ΓΘ
Θ®2Θ©Ζ÷Ή”bd2ΒΡΒγΉ” Ϋ______________Θ§‘ΣΥΊeΒΡΗΚ“ΜΦέ“θάκΉ”ΒΡΫαΙΙ Ψ“βΆΦ_________________ΓΘ
Θ®3Θ©Έο÷ bd2ΚΆhd2‘ΎΈοάμ–‘÷ …œ”–Ή≈Ψό¥σ≤ν“λΘ§Τδ‘≠“ρ «Έο÷ bd2 τ”Ύ_________________ΨßΧεΘ§Έο÷ hd2 τ”Ύ______________ΨßΧεΓΘ
Θ®4Θ©‘ΣΥΊeΒΡΤχΧ§«βΜ·Έο±»‘ΣΥΊiΒΡΤχΧ§«βΜ·Έο_________Θ®ΧνΓΑΈ»Ε®Γ±ΜρΓΑ≤ΜΈ»Ε®Γ±Θ©
Θ®5Θ©‘ΣΥΊfΓΔgΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΥ°Μ·Έο÷°ΦδΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ «_________________________ΘΜ
Θ®6Θ©Έο÷ fdaΚ§”–ΒΡΜ·―ßΦϋάύ–Ά «________________________________
Θ®Ι≤12Ζ÷Θ©
Θ®1Θ©κ≤ Ζζ «βΘ®ΟΩΩ’1Ζ÷Θ© Θ®2Θ© 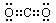
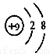 Θ®ΟΩΩ’1Ζ÷Θ©
Θ®ΟΩΩ’1Ζ÷Θ©
Θ®3Θ©Ζ÷Ή” ‘≠Ή” Θ®ΟΩΩ’1Ζ÷Θ© Θ®4Θ©Έ»Ε® Θ®1Ζ÷Θ©
Θ®5Θ©Al(OH)3+OH- = AlO2- +2H2O Θ®2Ζ÷Θ© Θ®6Θ©άκΉ”ΦϋΚΆΙ≤ΦέΦϋΘ®2Ζ÷Θ©
ΓΨΫβΈωΓΩ

 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ