��Ŀ����
����ͼװ�ã�
����֧�������ʢ�ŵĶ��ǹ����Լ���a��Ϊ��״��ɫ���壻B��Ϊdz��ɫ��״���ڲ������ϵ��Լ���C��Ϊ��ɫ����״�Ĺ��壮bΪ�ߴ̼�����ζ����Һ��b��a��B�е��Լ����ܷ�Ӧ��������C���Լ���Ӧ��������Щ�Լ����dz����ģ��ѵ�ȼ��������ڹ��ƿD�У�
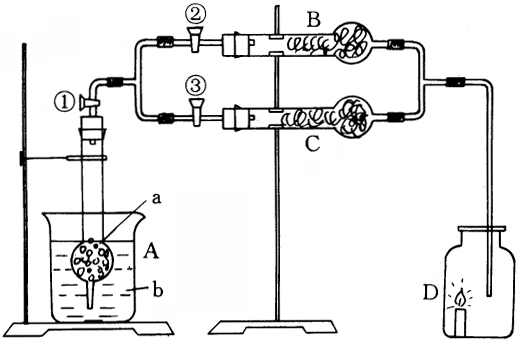
��������١��ڹرջ�����D������ȼ�ո�Ϊ��ʢ���dz���������ʱ�رջ����ڣ������ۣ���������Ϩ��
(1)д����ѧʽ��a________��b________��Һ��B��________��C��________��
(2)д��A�з�Ӧ�����ӷ���ʽ________��
(3)������ȡ��װ��A�������٣���Ӧ���У��رջ����٣����÷�Ӧֹͣ��Ϊʲô�������濪���ã������ͣ���������ԭ����________��
������
|
�𰸣� (1)a�� (2) (3)�����٣���Һ�������Ӵ�����ʯ������Ӧ������ ʹ����ȼ�ո������ǽ���������������Ϩ���ǽ����� |
��ʾ��
|
����װ�úͲ�ͬ�����IJ�ͬ����ж����ʺͷ�Ӧ�ǽ��곣����ʵ�����ͣ���ʱ�������ᴿ����ϵ��������Ҫ���������ҩƷ��״Ϊͻ�ƿ��жϣ���֮Ҳ�ɸ�Ϊ��֪ҩƷ���жϷ�Ӧ����������ͣ� |

Ϊ���о����������H2O2�ֽ����ʵ�Ӱ�죬ijͬѧ��������ʵ�飬��ش��������⡣
| ��� | ���� | ʵ������ |
| �� | �ֱ����Թ�A��B�м���5mL 5%H2O2��Һ��������2��1 mol/L FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��У����Թ�B����ʢ��40��������ˮ���ձ��С� | �Թ�A�в������������٣��Թ�B�в��������������� |
| �� | ��ȡ��֧�Թֱܷ����5mL 5%H2O2��Һ��5mL 10%H2O2��Һ | �Թ�A��B�о�δ���Լ��������ݲ����� |
��2��ʵ��ٵ�Ŀ����________��
��3��ʵ���δ�۲쵽Ԥ�ڵ�ʵ������Ϊ�˰�����ͬѧ�ﵽʵ��Ŀ�ģ�������Ķ����������ĸĽ������_______����ʵ�������ṩ�ļ����Լ�����
��4��ijͬѧ��50 mLһ��Ũ�ȵ�H2O2��Һ�м���һ�����Ķ������̣��ų�������������״���£��뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ����A��B��C��������ʾ��˲ʱ��Ӧ����������______���A����B����C������

��5������H2O2�ֽⷴӦ��Cu2+Ҳ��һ���Ĵ����á�Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣��ش�������⣺

�ٶ��Է�������ͼ��ͨ���۲�_________�����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2��SO4��3��Ϊ��������������_______��
�ڶ�����������ͼ����ʾװ��������ʵ�飬ʵ��ʱ��������40 mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ʵ������Ҫ������������_________��
��ij��ѧ�С���������ͼ��ʾ�����ּг�װ������ȥ��ʵ��װ�ã���̽����ʪ��Cl2�����Na2CO3 ���巴Ӧ�õ��Ĺ������ʵijɷ֡�
��֪��ͨ��һ�������������D��ֻ��һ�ֳ�����Ϊ�ƺ�ɫ�����壬��Ϊ�������������ȷ������C�й��庬��NaHCO3 ���Һ��ȵ���ֻ��һ�֡��ֶ�C�ijɷֽ��в����̽����

��1������������룺����֪C����0.1molCl2ǡ�ú�10.6��Na2CO3������ȫ��Ӧ����C����Cl2���뷴Ӧ�Ļ�ѧ����ʽ���� ��
��2��������������衣
����1���������ֳɷ֣�NaHCO3�� ��
����2���������ֳɷ֣�NaHCO3�� �� ��
����ƺ���������C�����е�δ֪�ɷֽ���̽������д��ʵ�鲽���Լ�Ԥ������ͽ��ۣ��ɲ���������
��ѡʵ���Լ�������������ˮ��ϡHNO3��Ba(OH)2��Һ��BaCl2��Һ������ʯ��ˮ��AgNO3��Һ���Թܡ�С�ձ���
|
ʵ�鲽�� |
Ԥ������ͽ��� |
|
����1��ȡ����C�й�����Ʒ���Թ��У�������������ˮ���������������ȫ�ܽ⣬Ȼ��������Һ��װA��B��֧�Թ��С� |
|
|
����2�� |
|
|
����3�� |
|
|
|
|
��̽������ʯ�����ĺ�����2. 25g����ʯ��Ʒ��һϵ�л�ѧ�������Ƶ���Ԫ��ȫ��ΪFe2���Ĵ���Һ250 mL�����÷�Ӧ 6Fe2����Cr2O72����14H����6Fe3����2Cr3����7H2O ������ʯ����Ԫ�صĺ������вⶨ��
�������ձ�������������Ͳ����ͷ�ιܣ�Ҫ����0.0150 mol/L K2Cr2O7��Һ100mL������Ҫ�IJ��������� ��
��ȡ25mL����Һ���еζ���ƽ������ K2Cr2O7��Һ���Ϊ25.00 mL��������ʯ����Ԫ�صİٷֺ����ǣ�Fe�����ԭ������Ϊ56�� ��
���ڱ�ʵ��ĵζ������У����в�����ʹ�ⶨ���ƫС���� ����д��ţ���
a��δ�ñ�K2Cr2O7��Һ��ϴ�ζ���
b����ƿ�м��������Һ���ټ�����ˮ
c����ƿ�ڵζ������о���ҡ����������Һ�彦��
Ϊ���о����������H2O2�ֽ����ʵ�Ӱ�죬ijͬѧ��������ʵ�飬��ش��������⡣
|
��� |
���� |
ʵ������ |
|
�� |
�ֱ����Թ�A��B�м���5mL 5%H2O2��Һ��������2��1 mol/L FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��У����Թ�B����ʢ��40��������ˮ���ձ��С� |
�Թ�A�в������������٣��Թ�B�в��������������� |
|
�� |
��ȡ��֧�Թֱܷ����5mL 5%H2O2��Һ��5mL 10%H2O2��Һ |
�Թ�A��B�о�δ���Լ��������ݲ����� |
��1����������ֽ�Ļ�ѧ����ʽΪ________��
��2��ʵ��ٵ�Ŀ����________��
��3��ʵ���δ�۲쵽Ԥ�ڵ�ʵ������Ϊ�˰�����ͬѧ�ﵽʵ��Ŀ�ģ�������Ķ����������ĸĽ������_______����ʵ�������ṩ�ļ����Լ�����
��4��ijͬѧ��50 mLһ��Ũ�ȵ�H2O2��Һ�м���һ�����Ķ������̣��ų�������������״���£��뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ����A��B��C��������ʾ��˲ʱ��Ӧ����������______���A����B����C������

��5������H2O2�ֽⷴӦ��Cu2+Ҳ��һ���Ĵ����á�Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣��ش�������⣺

�ٶ��Է�������ͼ��ͨ���۲�_________�����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2��SO4��3��Ϊ��������������_______��
�ڶ�����������ͼ����ʾװ��������ʵ�飬ʵ��ʱ��������40 mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ʵ������Ҫ������������_________��
Ϊ���о���������Թ�������ֽ����ʵ�Ӱ�죬ijͬѧ��������ʵ�飬��ش��������⡣
| ��� | ���� | ʵ������ |
| �� | �ֱ����Թ�A��B�м���5 mL 5% H2O2��Һ��������2��1 mol/L FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��н��ݣ����Թ�B����ʢ��40��������ˮ���ձ��н��ݡ� | �Թ�A�в��ٲ������ݣ� �Թ�B�в��������������� |
| �� | ��ȡ��֧�Թֱܷ����5 mL 5% H2O2��Һ��5 mL 10% H2O2��Һ | �Թ�A��B�о�δ���Լ��������ݲ����� |
��1����������ֽ�Ļ�ѧ����ʽΪ �� ��1�֣���
��2��ʵ��ٵ�Ŀ���� �� ��1�֣���
ʵ���еμ�FeCl3��Һ��Ŀ���� �� ��1�֣���
��3��ʵ���δ�۲쵽Ԥ�ڵ�ʵ������Ϊ�˰�����ͬѧ�ﵽʵ��Ŀ�ģ�������Ķ����������ĸĽ������ �� ��2�֣�����ʵ�������ṩ�ļ����Լ�����
��4��ijͬѧ��50 mLһ��Ũ�ȵ�H2O2��Һ�м���һ�����Ķ������̣��ų�������������״���£��뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ����A��B��C��������ʾ��˲ʱ��Ӧ������������ �� ��1�֣���

��5������H2O2�ֽⷴӦ��Cu2+Ҳ��һ���Ĵ����á�Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣��ش�������⣺

�ٶ��Է�������ͼ��ͨ���۲� �� ��1�֣������ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ�������������� �� ��2�֣���
�ڶ�����������ͼ����ʾװ��������ʵ�飬ʵ��ʱ��������40 mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ʵ������Ҫ������������ �� ��2�֣���