��Ŀ����
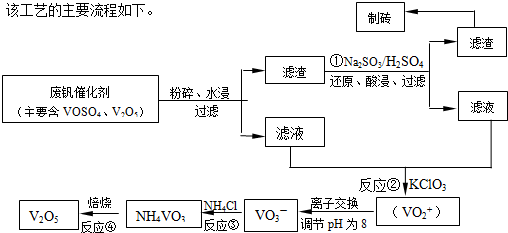
����Ͽ�ѧ�ķ�չ�����������仯����õ���Խ��Խ�㷺��Ӧ�ã�Ϊ�������ú�������������V2O5��VOSO4�������Բ�������������Ա����������һ�����ӽ��������շ����¹��գ������ʴ�91.7%���ϣ��ù��յ���Ҫ������ͼ1��
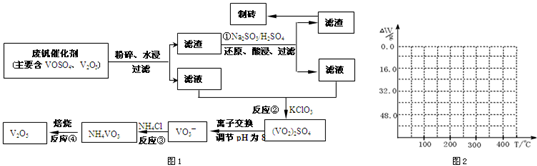
���ַ��Ļ�������ˮ�е��ܽ������±���ʾ���ɴ˻ش��������⣺
��1��23V��Ԫ�����ڱ�λ�ڵ�______����______�壮��ҵ����V2O5ұ���������������ȼ������仯ѧ����ʽΪ______��
��2����Ӧ�ٵ�Ŀ����______��
��3���ⶨ��Ӧ����Һ�з��ĺ�����������֪Ũ�ȵ��ữH2C2O4��Һ�ζ���VO2��2SO4��Һ����Ҫ����ΪCO2��VOSO4�������ӷ���ʽΪ��______��
��4����֪NH4VO3�ڱ��չ�����150��200��ʱ��ʧȥ������300��350����ʧȥˮ�������ұ�����ͼ2�л�������234g NH4VO3���������ļ���ֵ��W���¶ȣ�T���仯�����ߣ�

���ַ��Ļ�������ˮ�е��ܽ������±���ʾ���ɴ˻ش��������⣺
| ���� | VOSO4 | V2O5 | NH4VO3 | ��VO2��2SO4 |
| �ܽ��� | ���� | ���� | ���� | ���� |
��2����Ӧ�ٵ�Ŀ����______��
��3���ⶨ��Ӧ����Һ�з��ĺ�����������֪Ũ�ȵ��ữH2C2O4��Һ�ζ���VO2��2SO4��Һ����Ҫ����ΪCO2��VOSO4�������ӷ���ʽΪ��______��
��4����֪NH4VO3�ڱ��չ�����150��200��ʱ��ʧȥ������300��350����ʧȥˮ�������ұ�����ͼ2�л�������234g NH4VO3���������ļ���ֵ��W���¶ȣ�T���仯�����ߣ�
��1������23V��������������Ϊ23��ԭ�ӣ����Է�Ԫ��λ�ڵ������ڣ��� VB ���壻������ȷ�Ӧ��ԭ��д����ѧ����ʽ���ʴ𰸣��ģ�VB�� 3V2O5+10A
l6V+5Al2O3��
��2������������������ƣ�Ŀ��������������ԭ��Ӧ�����������ƻ�ԭV2O5����V2O5 ת��Ϊ�����Ե�VOSO4�������ᴿ���ʴ𰸣���V2O5 ת��Ϊ�����Ե�VOSO4��
��3���ⶨ��Ӧ����Һ�з��ĺ�����������֪Ũ�ȵ��ữH2C2O4��Һ�ζ���VO2��2SO4��Һ����Ҫ����ΪCO2��VOSO4�����ݻ��ϼ�������д�����ӷ���Ϊ2VO2++H2C2O4+2H+=2 VO2++2 CO2��+2 H2O��
�ʴ𰸣�2VO2++H2C2O4+2H+=2 VO2++2 CO2��+2 H2O��
��4����ʽΪ��
2NH4VO3�TV2O5+2NH3��+H2O
234g 34g 18g
����ֵ��ʼΪ0��34g�����ߴ�150�濪ʼ���٣�34gNH3������200��ʱ����32.0g�����������߿�ʼƽֱ����300��ʱ�ֿ�ʼ���٣�H2O������������350��ʱ����52gʱ����48.0g�Եͣ��Ͳ��ٱ仯������ͼ������
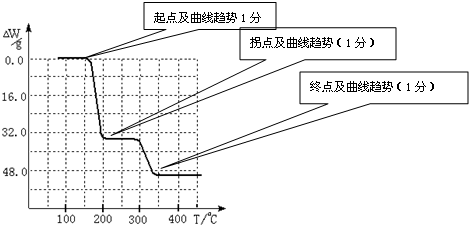
�ʴ�Ϊ��

| ||
��2������������������ƣ�Ŀ��������������ԭ��Ӧ�����������ƻ�ԭV2O5����V2O5 ת��Ϊ�����Ե�VOSO4�������ᴿ���ʴ𰸣���V2O5 ת��Ϊ�����Ե�VOSO4��
��3���ⶨ��Ӧ����Һ�з��ĺ�����������֪Ũ�ȵ��ữH2C2O4��Һ�ζ���VO2��2SO4��Һ����Ҫ����ΪCO2��VOSO4�����ݻ��ϼ�������д�����ӷ���Ϊ2VO2++H2C2O4+2H+=2 VO2++2 CO2��+2 H2O��
�ʴ𰸣�2VO2++H2C2O4+2H+=2 VO2++2 CO2��+2 H2O��
��4����ʽΪ��
2NH4VO3�TV2O5+2NH3��+H2O
234g 34g 18g
����ֵ��ʼΪ0��34g�����ߴ�150�濪ʼ���٣�34gNH3������200��ʱ����32.0g�����������߿�ʼƽֱ����300��ʱ�ֿ�ʼ���٣�H2O������������350��ʱ����52gʱ����48.0g�Եͣ��Ͳ��ٱ仯������ͼ������

�ʴ�Ϊ��


��ϰ��ϵ�д�
 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
�����Ŀ
����Ͽ�ѧ�ķ�չ�����������仯����õ���Խ��Խ�㷺��Ӧ�ã�Ϊ�������ú�������������V2O5��VOSO4�������Բ�������������Ա����������һ�����ӽ��������շ����¹��գ������ʴ�91.7%���ϣ��ù��յ���Ҫ������ͼ1��
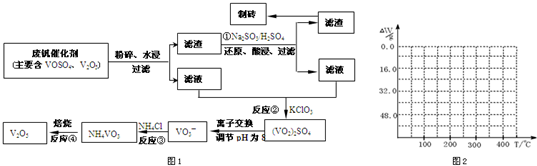
���ַ��Ļ�������ˮ�е��ܽ������±���ʾ���ɴ˻ش��������⣺
| ���� | VOSO4 | V2O5 | NH4VO3 | ��VO2��2SO4 |
| �ܽ��� | ���� | ���� | ���� | ���� |
��2����Ӧ�ٵ�Ŀ����______��
��3���ⶨ��Ӧ����Һ�з��ĺ�����������֪Ũ�ȵ��ữH2C2O4��Һ�ζ���VO2��2SO4��Һ����Ҫ����ΪCO2��VOSO4�������ӷ���ʽΪ��______��
��4����֪NH4VO3�ڱ��չ�����150��200��ʱ��ʧȥ������300��350����ʧȥˮ�������ұ�����ͼ2�л�������234g NH4VO3���������ļ���ֵ��W���¶ȣ�T���仯�����ߣ�
����Ͽ�ѧ�ķ�չ�����������仯����õ���Խ��Խ�㷺��Ӧ�ã�Ϊ�������ú�������������V2O5��VOSO4�������Բ�������������Ա����������һ�����ӽ��������շ����¹��գ������ʴ�91.7%���ϣ��ù��յ���Ҫ������ͼ1��
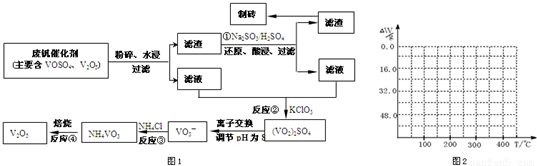
���ַ��Ļ�������ˮ�е��ܽ������±���ʾ���ɴ˻ش��������⣺
��1��23V��Ԫ�����ڱ�λ�ڵ�______����______�壮��ҵ����V2O5ұ���������������ȼ������仯ѧ����ʽΪ______ l6V+5Al2O3

���ַ��Ļ�������ˮ�е��ܽ������±���ʾ���ɴ˻ش��������⣺
| ���� | VOSO4 | V2O5 | NH4VO3 | ��VO2��2SO4 |
| �ܽ��� | ���� | ���� | ���� | ���� |

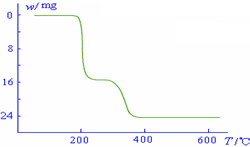 ������������ͼ��ʾ����NH4VO3�ڷֽ������
������������ͼ��ʾ����NH4VO3�ڷֽ������