��Ŀ����
J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����±���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ��

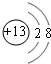
(1)M�����ӽṹʾ��ͼΪ________��Ԫ��T�����ڱ���λ�ڵ�________�塣
(2)J������ɵĻ����������6��ԭ�ӣ���ṹ��ʽΪ________��
(3)M��T�γɵĻ������ڳ�ʪ�Ŀ�����ð��ɫ��������Ӧ�Ļ�ѧ����ʽΪ____________________��
(4)L�������̬�⻯���ˮ��Һ�Լ��ԡ�
�������ӹ�ҵ�У���ˮ��Һ������ʴ��H2O2�����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ______________________��
��һ�������£����ڹ̶�������ܱ������з����ֽⷴӦ(��H>0)����ƽ����ı��±��з�Ӧ����x����ƽ����ϵ����x����y�ݼ�����________(ѡ�����)��
(2)J������ɵĻ����������6��ԭ�ӣ���ṹ��ʽΪ________��
(3)M��T�γɵĻ������ڳ�ʪ�Ŀ�����ð��ɫ��������Ӧ�Ļ�ѧ����ʽΪ____________________��
(4)L�������̬�⻯���ˮ��Һ�Լ��ԡ�
�������ӹ�ҵ�У���ˮ��Һ������ʴ��H2O2�����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ______________________��
��һ�������£����ڹ̶�������ܱ������з����ֽⷴӦ(��H>0)����ƽ����ı��±��з�Ӧ����x����ƽ����ϵ����x����y�ݼ�����________(ѡ�����)��

(5)��J��R�γɵ�Һ̬������JR2 0.2 mol��O2����ȫȼ�գ�����������̬�����298 Kʱ�ų�����215
kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ_________________________��
kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ_________________________��
(1) ����A��
����A��
(2)CH2=CH2
(3)AlCl3��3H2O==Al(OH)3��3HCl��
(4)��2NH3��H2O��3H2O2==N2����8H2O��2NH3��3H2O2===N2����6H2O����ac
(5)
 ����A��
����A��(2)CH2=CH2
(3)AlCl3��3H2O==Al(OH)3��3HCl��
(4)��2NH3��H2O��3H2O2==N2����8H2O��2NH3��3H2O2===N2����6H2O����ac
(5)


��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
 ��2010?������J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
��2010?������J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
 Al��OH��3+3HCl
Al��OH��3+3HCl
 J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
 J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�������JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�
J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�������JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�