��Ŀ����
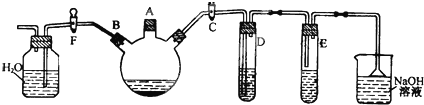
ij�����о���ѧϰС������ͼ��ʾװ���Ʊ������屽����֤���뱽�ķ�Ӧ��ȡ����Ӧ��

ʵ��ʱ���ر�F��������C��������װ����������������ƿ����A�ڼ��������壬�ټ�����м����סA�ڣ�
�ش��������⣺
��1��D�Թ���װ����ʲô______����������______��
��2��E�Թ���װ����______
��3����ȥ�屽�л��е�Br2���ʵ��Լ���______����������Ϊ______��
��4��������ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ______��______��
��5������ʲô�����֤����֤���뱽�ķ�Ӧ��ȡ����Ӧ��______��

ʵ��ʱ���ر�F��������C��������װ����������������ƿ����A�ڼ��������壬�ټ�����м����סA�ڣ�
�ش��������⣺
��1��D�Թ���װ����ʲô______����������______��
��2��E�Թ���װ����______
��3����ȥ�屽�л��е�Br2���ʵ��Լ���______����������Ϊ______��
��4��������ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ______��______��
��5������ʲô�����֤����֤���뱽�ķ�Ӧ��ȡ����Ӧ��______��
��1�����ڷ�Ӧ���ȣ�����Һ����ӷ��������弫���������Ȼ�̼�������Ȼ�̼��CCl4����ȥ�廯�������е��������ͱ����Է����ż���H+��Br-��
�ʴ�Ϊ��CCl4����ȥ�ӷ��������壻
��2��E�Թ���װ���Լ�������Ϊ�������ɵ��廯�⣬���������廯�������������Ӻ������ӷ�Ӧ��AgNO3+HBr=AgBr�����ɵ���ɫ����������ij����廯�����ɴ���������ͱ�������ȡ����Ӧ��
�ʴ�Ϊ����������Һ��
��3��2NaOH+Br2�TNaBr+NaBrO+H2O�����Գ�ȥ�屽�л��е�Br2���ʵ��Լ����������ƣ�NaBr �� NaBrO���屽�����ܣ�������������ƿ�м�����������������Һ����ת���Һ©������Һ���ɣ�
�ʴ�Ϊ������������Һ����������ƿ�м�����������������Һ����ת���Һ©������Һ��
��4���ڴ����������£������巴Ӧ�������廯�������廯���뱽���ã������屽��ͬʱ���廯�����ɣ�
�ʴ�Ϊ��2Fe+3Br2=2FeBr3��C6H6+Br2
C6H5Br+HBr��
��5���ڴ����������£������ϵ���ԭ�ӱ���ԭ����ȡ���������屽��ͬʱ���廯�����ɣ�E�Թ���װ���Լ�������Ϊ�������ɵ��廯�⣬�����廯�������������Ӻ������ӷ�Ӧ�����ɵ���ɫ����������ij����廯�����ɴ���������ͱ�������ȡ����Ӧ��
�ʴ�Ϊ��E�в�������ɫ������
�ʴ�Ϊ��CCl4����ȥ�ӷ��������壻
��2��E�Թ���װ���Լ�������Ϊ�������ɵ��廯�⣬���������廯�������������Ӻ������ӷ�Ӧ��AgNO3+HBr=AgBr�����ɵ���ɫ����������ij����廯�����ɴ���������ͱ�������ȡ����Ӧ��
�ʴ�Ϊ����������Һ��
��3��2NaOH+Br2�TNaBr+NaBrO+H2O�����Գ�ȥ�屽�л��е�Br2���ʵ��Լ����������ƣ�NaBr �� NaBrO���屽�����ܣ�������������ƿ�м�����������������Һ����ת���Һ©������Һ���ɣ�
�ʴ�Ϊ������������Һ����������ƿ�м�����������������Һ����ת���Һ©������Һ��
��4���ڴ����������£������巴Ӧ�������廯�������廯���뱽���ã������屽��ͬʱ���廯�����ɣ�
�ʴ�Ϊ��2Fe+3Br2=2FeBr3��C6H6+Br2
| FeBr3 |
��5���ڴ����������£������ϵ���ԭ�ӱ���ԭ����ȡ���������屽��ͬʱ���廯�����ɣ�E�Թ���װ���Լ�������Ϊ�������ɵ��廯�⣬�����廯�������������Ӻ������ӷ�Ӧ�����ɵ���ɫ����������ij����廯�����ɴ���������ͱ�������ȡ����Ӧ��
�ʴ�Ϊ��E�в�������ɫ������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


 ��װ���� ���������� ��
��װ���� ���������� ��