��Ŀ����
�ݱ�����Ħ��������˾������һ���Լ״�Ϊԭ�ϣ���KOHΪ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���¡���ͼ��һ���绯ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH
����գ�
(1)���ʱ����ԭ��صĸ������Դ________���������������ĵ缫��ӦΪ_____________��
(2)�ŵ�ʱ�������ĵ缫��ӦʽΪ__________________________________________��
(3)�ڴ˹���������ȫ��Ӧ���ҳ���A������������648 g����׳�������������O2_______L��(��״����)
(4)���ڳ��³�ѹ�£�1 g CH3OHȼ������CO2��Һ̬H2Oʱ����22.68 kJ,��ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ ________________________��
���������ʱ��ʹ��Ӧ��������У�����Ҫ����O2������Ҫ����CH3OH���ֱ���������Ӧ�ͻ�ԭ��Ӧ���ŵ�ʱ��������CH3OH�ŵ磬����������Ӧ��
���ݵ��ӵ�ʧ�غ㣬��
4Ag![]() O2
O2
![]() V(O2)
V(O2)
��V(O2)=33.6 L��
�𰸣�(1)�ٸ� ��4OH--4e-====2H2O+O2��
(2)CH3OH-6e-+8OH-====![]() +6H2O
+6H2O
(3)33.6
(4)CH3OH(l)+![]() O2(g) ====CO2(g)+2H2O(l);��H=-725.80 kJ��mol-1
O2(g) ====CO2(g)+2H2O(l);��H=-725.80 kJ��mol-1

��ϰ��ϵ�д�
�����Ŀ
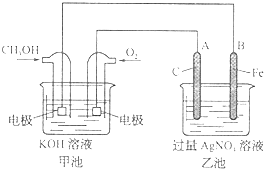 �ݱ�����Ħ��������˾������һ���Լ״�Ϊԭ�ϣ���KOHΪ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���£���ͼ��һ���绯ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH
�ݱ�����Ħ��������˾������һ���Լ״�Ϊԭ�ϣ���KOHΪ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���£���ͼ��һ���绯ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH Ϊ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���¡���ͼ��һ���绯ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH
Ϊ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���¡���ͼ��һ���绯ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH 2K2CO3+6H2O
2K2CO3+6H2O
 _________L����״���£���
_________L����״���£��� Ϊ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���¡���ͼ��һ���绯ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH
Ϊ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���¡���ͼ��һ���绯ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH 2K2CO3+6H2O
2K2CO3+6H2O
 _________L����״���£���
_________L����״���£���