��Ŀ����
12��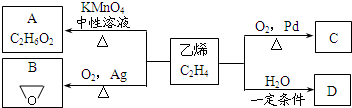 ��ϩ������ת����ϵ����ϩ�ڲ�ͬ�������¿ɱ������ɲ�ͬ�����A��B��C����֪��ȡ0.01mol A������������ȫ��Ӧ������224mL����״�������壮C��B��ͬ���칹�壬C�����Ƶ�������ͭ��Һһ����ȣ��������ɫ������
��ϩ������ת����ϵ����ϩ�ڲ�ͬ�������¿ɱ������ɲ�ͬ�����A��B��C����֪��ȡ0.01mol A������������ȫ��Ӧ������224mL����״�������壮C��B��ͬ���칹�壬C�����Ƶ�������ͭ��Һһ����ȣ��������ɫ���������������գ�
��1����ҵ����ʯ�͵õ���ϩ�ķ�����C��
A������ B������ C���ѽ�D���ѻ�
��2��A�й����ŵ�����Ϊ�ǻ���д��C�Ľṹ��ʽCH3CHO��
��3����ϩ��D�Ļ�ѧ����ʽΪCH2=CH2+H2O$\stackrel{һ������}{��}$CH3CH2OH����Ӧ�����Ǽӳɷ�Ӧ��
��4��һ�������£�B����H2O��������A��д���ù��̵Ļ�ѧ����ʽ
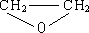 +H2O$\stackrel{һ������}{��}$
+H2O$\stackrel{һ������}{��}$ ��
��
���� 0.01molA�������Ľ�������ȫ���ú�����224mL����״�������壬����������Ϊ$\frac{0.224L}{22.4L/mol}$=0.01mol�����A�ķ���ʽ��֪��A�����к���2���ǻ�����AΪ�Ҷ������ṹ��ʽΪ ��C��B��ͬ���칹�壬C�����Ƶ�������ͭ��Һһ����ȣ��������ɫ��������C����ȩ������CΪCH3CHO����ϩ��ˮ�����ӳɷ�Ӧ����DΪCH3CH2OH���ݴ˽��
��C��B��ͬ���칹�壬C�����Ƶ�������ͭ��Һһ����ȣ��������ɫ��������C����ȩ������CΪCH3CHO����ϩ��ˮ�����ӳɷ�Ӧ����DΪCH3CH2OH���ݴ˽��
��� �⣺0.01molA�������Ľ�������ȫ���ú�����224mL����״�������壬����������Ϊ$\frac{0.224L}{22.4L/mol}$=0.01mol�����A�ķ���ʽ��֪��A�����к���2���ǻ�����AΪ�Ҷ������ṹ��ʽΪ ��C��B��ͬ���칹�壬C�����Ƶ�������ͭ��Һһ����ȣ��������ɫ��������C����ȩ������CΪCH3CHO����ϩ��ˮ�����ӳɷ�Ӧ����DΪCH3CH2OH��
��C��B��ͬ���칹�壬C�����Ƶ�������ͭ��Һһ����ȣ��������ɫ��������C����ȩ������CΪCH3CHO����ϩ��ˮ�����ӳɷ�Ӧ����DΪCH3CH2OH��
��1����ҵ��ͨ��ʯ���ѽ�õ���ϩ����ѡ��C��
��2��A�ṹ��ʽΪ �������ŵ�����Ϊ�ǻ���C�Ľṹ��ʽΪCH3CHO��
�������ŵ�����Ϊ�ǻ���C�Ľṹ��ʽΪCH3CHO��
�ʴ�Ϊ���ǻ���CH3CHO��
��3����ϩ��D�Ļ�ѧ����ʽΪ��CH2=CH2+H2O$\stackrel{һ������}{��}$CH3CH2OH�����ڼӳɷ�Ӧ��
�ʴ�Ϊ��CH2=CH2+H2O$\stackrel{һ������}{��}$CH3CH2OH���ӳɷ�Ӧ��
��4��һ�������£�B����H2O��������A���ù��̵Ļ�ѧ����ʽΪ��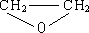 +H2O$\stackrel{һ������}{��}$
+H2O$\stackrel{һ������}{��}$ ��
��
�ʴ�Ϊ��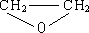 +H2O$\stackrel{һ������}{��}$
+H2O$\stackrel{һ������}{��}$ ��
��
���� ���⿼���л����ƶϣ��漰ϩ��������ȩ��������ת��������ȷ��AΪ�Ҷ����ǹؼ����ѶȲ���

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�| A�� | ������ѹǿ���� | B�� | KO2�������ֲ��� | C�� | ƽ�ⳣ����С | D�� | ����Ũ�Ȳ��� |
| A�� | BH3 | B�� | SiH4 | C�� | C2H2 | D�� | NaH |
| A�� | ���������л�ԭ�ԣ������������� | B�� | FeCl3�������ԣ���������ӡˢ��· | ||
| C�� | ���л�ԭ�ԣ���ұ��ijЩ���� | D�� | Ũ��������ˮ�ԣ������ڸ��ﰱ�� |
| A | B | C | D | |
| ���� | Һ�� | ̼��� | �Ȼ��� | Ũ���� |
| ��; | ����� | ����ҩ | ������ | ��ʴ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
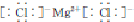 ����ҵ���õ����ˮ�Ȼ�þ����������þ���þ��ԭ�������Ĵ����ĵ��ܣ�
����ҵ���õ����ˮ�Ȼ�þ����������þ���þ��ԭ�������Ĵ����ĵ��ܣ� ��g��+3H2��g�� $?_{FeSO_{4}/Al_{2}O_{3}}^{����}$
��g��+3H2��g�� $?_{FeSO_{4}/Al_{2}O_{3}}^{����}$ ��g��
��g�� 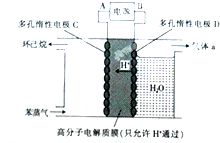
 ����CH3CH2Br+NaOH$��_{��}^{��}$CH2=CH2+NaBr+H2O����CH3-CH2Br+NaOH$��_{��}^{H_{2}O}$CH3-CH2OH+NaBr��
����CH3CH2Br+NaOH$��_{��}^{��}$CH2=CH2+NaBr+H2O����CH3-CH2Br+NaOH$��_{��}^{H_{2}O}$CH3-CH2OH+NaBr��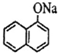 ���Լ�b�й����ŵ���������ԭ�ӡ�̼̼˫�����۵ķ�Ӧ������������Ӧ��
���Լ�b�й����ŵ���������ԭ�ӡ�̼̼˫�����۵ķ�Ӧ������������Ӧ�� ����F����һ��������Ļ�ѧ����ʽΪ
����F����һ��������Ļ�ѧ����ʽΪ +HNO3��Ũ��$��_{��}^{Ũ����}$
+HNO3��Ũ��$��_{��}^{Ũ����}$ +H2O��
+H2O��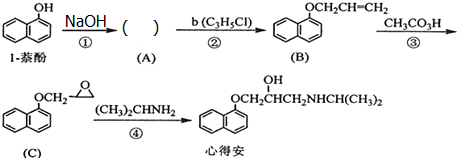


 �����ķ����к���4�ֲ�ͬ��ѧ��������ԭ�ӣ�
�����ķ����к���4�ֲ�ͬ��ѧ��������ԭ�ӣ� $��_{��}^{Ũ����}$
$��_{��}^{Ũ����}$ +H2O��
+H2O�� ��
��