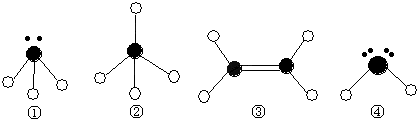
��Ŀ����
 A��B��C��D�Ƕ�����Ԫ���γɵ��������嵥�ʣ�E��F��Ϊ���壬��FΪ����ɫ���йص�ת����ϵ��ͼ��ʾ����Ӧ����������ȥ����
A��B��C��D�Ƕ�����Ԫ���γɵ��������嵥�ʣ�E��F��Ϊ���壬��FΪ����ɫ���йص�ת����ϵ��ͼ��ʾ����Ӧ����������ȥ������ش��������⣺
��1��D�Ļ�ѧʽΪ
��2����Ӧ�۵����ӷ���ʽΪ
��3��Y��E��һ�������¿ɷ�Ӧ����B��Z������һ������ʵ������ķ�Ӧ��������E�Ի�������Ⱦ���÷�Ӧ�Ļ�ѧ����ʽΪ
��4��0.1mol?L-1��X��Һ��0.1mol?L-1��Y��Һ�������ϣ���Һ��
��5��������0.1mol?L-1��Y��Һ��c��H+��/c��OH-��=1��10-8������������ȷ����
A������Һ��pH=11
B������Һ�е����ʵ������������Ũ��0.1mol?L-1
C������Һ��ˮ�������c��H+����c��OH-���˻�Ϊ1��10-22
D��pH=3��������ҺV1 L���0.1mol?L-1��Y��ҺV2 L��ϣ��������ҺpH=7����V1��V2
E����������Һ��ˮϡ��100����pHΪ9��
���ƿ�֪EΪNO��CΪO2��ZΪH2O��YΪNH3��XΪHCl��GΪHNO3��
��2����ΪNO2��H2O�ķ�Ӧ��
��3�����漰��Ӧ������Ϊ������ԭ��Ӧ����ԭ����������ԭ�еĹ��з�Ӧ��
��4������ǡ����ȫ��Ӧʱ����NH4Cl��笠�����ˮ���ʹ��Һ�����ԣ�
��5��������Kw=c��H+����c��OH-��=1��10-14�����������Ϣ
| c(H+) |
| c(OH-) |
 NH4++OH-�ɵ��ɰ�ˮ�������c��NH4+��=1��10-3 mol?L-1��B������ˮ�������c��H+��=c��OH-��=1��10-11mol?L-1��C��ȷ��ѡ��D��Ȼ����ȷ��ѡ��E���ǵ���ˮ���������Һ���ʼ�ˮϡ��100������Һ��pH����9��
NH4++OH-�ɵ��ɰ�ˮ�������c��NH4+��=1��10-3 mol?L-1��B������ˮ�������c��H+��=c��OH-��=1��10-11mol?L-1��C��ȷ��ѡ��D��Ȼ����ȷ��ѡ��E���ǵ���ˮ���������Һ���ʼ�ˮϡ��100������Һ��pH����9���ʴ�Ϊ��H2��
��2����ΪNO2��H2O�ķ�Ӧ����Ӧ�����ӷ���ʽΪ3NO2+H2O=2H++2NO3-+NO��
�ʴ�Ϊ��3NO2+H2O=2H++2NO3-+NO��
��3��EΪNO��Ϊ��Ⱦ�����壬��һ�������¿���NH3��Ӧ����N2��H2O����Ӧ�Ļ�ѧ����ʽΪ4NH3+6NO=5N2+6H2O��
�ʴ�Ϊ��4NH3+6NO=5N2+6H2O��
��4������ǡ����ȫ��Ӧʱ����NH4Cl��Ϊǿ�������Σ���Һ�д���NH4++H2O
 NH3?H2O+H+��笠�����ˮ���ʹ��Һ�����ԣ�
NH3?H2O+H+��笠�����ˮ���ʹ��Һ�����ԣ��ʴ�Ϊ���NH4++H2O
 NH3?H2O+H+��
NH3?H2O+H+����5��A��������Kw=c��H+����c��OH-��=1��10-14�����������Ϣ
| c(H+) |
| c(OH-) |
B������NH3?H2O
 NH4++OH-�ɵ��ɰ�ˮ�������c��NH4+��=1��10-3 mol?L-1����B����
NH4++OH-�ɵ��ɰ�ˮ�������c��NH4+��=1��10-3 mol?L-1����B����C����ˮ�������c��H+��=c��OH-��=1��10-11mol?L-1���ɵø���Һ��ˮ�������c��H+����c��OH-���˻�Ϊ1��10-22����C��ȷ��
D����ˮΪ������ʣ������Ũ�Ƚ�С����������ϣ���ˮ��������Һ�ʼ��ԣ�pH=7�������������ϴ�D��ȷ��
E�����ǵ���ˮ���������Һ���ʼ�ˮϡ��100������Һ��pH����9����E����
�ʴ�Ϊ��ACD��

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д�����ѧһѡ��3�����ʽṹ�����ʡ���15�֣�
��������Ԫ�أ�����A��B��C��DΪ����������Ԫ�أ�E��FΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮
| AԪ��ԭ�ӵĺ���p����������s����������1 |
| BԪ��ԭ�Ӻ���s����������p����������ȣ��Ҳ���AԪ����ͬһ���� |
| Cԭ�Ӻ�������p���ȫ������� |
| DԪ�ص������������������IJ�Ϊ4 |
| E��ǰ�������е縺����С��Ԫ�� |
| F�����ڱ��ĵ����� |
��
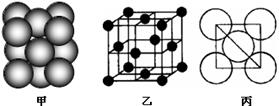
��2��ijͬѧ����������Ϣ��������B�����Ų�ͼ��ͼ

Υ���� ԭ����
��3��Fλ�� �� �������̬ԭ���� ���˶�״̬��
��4��CD3 ����ԭ�ӵ��ӻ���ʽΪ ���ü۲���ӶԻ��������Ʋ�����ӿռ乹��Ϊ ������EԪ�صķ�����
��5����ij�������ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ�����и�ԭ�ӵ���λ��Ϊ ���õ��ʾ�����ԭ�ӵĶѻ���ʽΪ���ֻ����ѻ���ʽ�е� ��
��������Ԫ�أ�����A��B��C��DΪ����������Ԫ�أ�E��FΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⡣
| AԪ��ԭ�ӵĺ���p����������s����������1 |
| BԪ��ԭ�Ӻ���s����������p����������ȣ��Ҳ���AԪ����ͬһ���� |
| Cԭ�Ӻ�������p���ȫ������� |
| DԪ�ص������������������IJ�Ϊ4 |
| E��ǰ�������е縺����С��Ԫ�� |
| F�����ڱ��ĵ����� |
��ijͬѧ����������Ϣ��������B�����Ų�ͼ��ͼ
 ��Υ���� ԭ����
��Υ���� ԭ������Fλ�� �� �������̬ԭ���� ���˶�״̬��
��CD3 ����ԭ�ӵ��ӻ���ʽΪ ���ü۲���ӶԻ��������Ʋ�����ӿռ乹��Ϊ ������EԪ�صķ����� ��
����ij�������ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ�����и�ԭ�ӵ���λ��Ϊ ���õ��ʾ�����ԭ�ӵĶѻ���ʽΪ���ֻ����ѻ���ʽ�е� ������֪�ý�����ԭ�Ӱ뾶Ϊd cm��NA���������ӵ����������������ԭ������ΪM����þ�����ܶ�Ϊ______g��cm��3(����ĸ��ʾ)��

����ѧһѡ��3�����ʽṹ�����ʡ���15�֣�
��������Ԫ�أ�����A��B��C��DΪ����������Ԫ�أ�E��FΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮
|
AԪ��ԭ�ӵĺ���p����������s����������1 |
|
BԪ��ԭ�Ӻ���s����������p����������ȣ��Ҳ���AԪ����ͬһ���� |
|
Cԭ�Ӻ�������p���ȫ������� |
|
DԪ�ص������������������IJ�Ϊ4 |
|
E��ǰ�������е縺����С��Ԫ�� |
|
F�����ڱ��ĵ����� |
��1��A��̬ԭ����������ߵĵ��ӣ���������ڿռ��� ������ԭ�ӹ����
��
��2��ijͬѧ����������Ϣ��������B�����Ų�ͼ��ͼ
Υ���� ԭ����
��3��Fλ�� �� �������̬ԭ���� ���˶�״̬��
��4��CD3 ����ԭ�ӵ��ӻ���ʽΪ ���ü۲���ӶԻ��������Ʋ�����ӿռ乹��Ϊ ������EԪ�صķ�����
��5����ij�������ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ�����и�ԭ�ӵ���λ��Ϊ ���õ��ʾ�����ԭ�ӵĶѻ���ʽΪ���ֻ����ѻ���ʽ�е� ��