��Ŀ����
����A��B��C��D4��Ԫ�أ�AԪ���γ�-2�������ӱȺ��ĺ�����Ӷ�8����BԪ�ص�һ��������Ϊ����ɫ���壬�ù�����������������A�ĵ��ʣ�CΪԭ�Ӻ�����12�����ӵĶ��۽�������2.4gC��������ˮ��Ӧʱ���ڱ�״���·ų�H22.24L��D��M������7�����ӡ�
��1��д��A��B��C��D4��Ԫ�صķ���________��________��________��________��
��2��д��B��C��D����������ˮ����Ļ�ѧʽ��________��________��________�����Ƚϼ���________________________________________��
��3��D����̬�⻯��Ϊ________��D����̬�⻯����H2S��HF���ȶ�����ǿ������˳��Ϊ_____________________________________________________________________��
�𰸣�
������
������
��1��O Na Mg Cl ��2��NaOH Mg(OH)2 HClO4 ���ԣ�NaOH>Mg(OH)2>HClO4 ��3��HCl HF>HCl>H2S
|

��ϰ��ϵ�д�
�����Ŀ
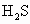 ��HF���ȶ�����ǿ������˳��Ϊ________��
��HF���ȶ�����ǿ������˳��Ϊ________��