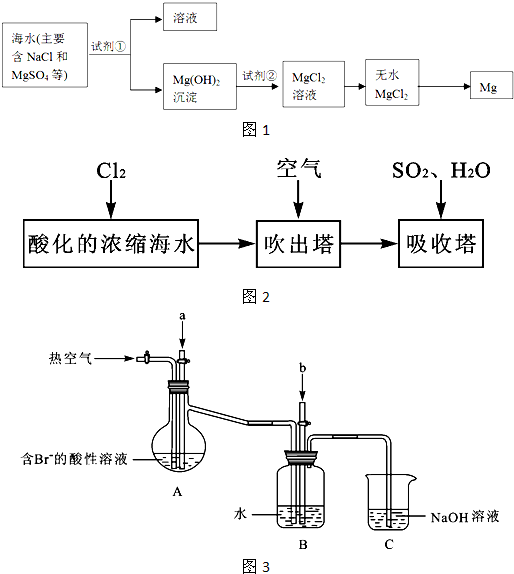
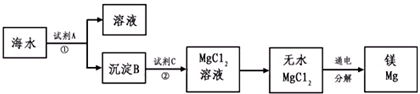
��Ŀ����
þ����Ͻ���һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ģ���Ҫ��������ͼ��ʾ

(1)Ϊ��ʹMgSO4ת��ΪMg(OH)2���Լ��ٿ���ѡ��__________________��MgSO4��ȫת��Ϊ����������ٵ���Ӧ_______________��
(2)�����Լ��ٺ��ܹ�����õ�Mg(OH)2�����ķ�����________��
(3)��ˮMgCl2������״̬�£���εõ�����þ��____________________��
(4)д���١������������з�����Ӧ�����ӷ���ʽ��_____________________________��
(2)�����Լ��ٺ��ܹ�����õ�Mg(OH)2�����ķ�����________��
(3)��ˮMgCl2������״̬�£���εõ�����þ��____________________��
(4)д���١������������з�����Ӧ�����ӷ���ʽ��_____________________________��
(1)Ca(OH)2������
(2)����
(3)��ⷨ��MgCl2�����ڣ� Mg+Cl2��
Mg+Cl2��
(4)��Mg2++2OH-=Mg(OH)2������Mg(OH)2+2H+=Mg2++2H2O
(2)����
(3)��ⷨ��MgCl2�����ڣ�
 Mg+Cl2��
Mg+Cl2�� (4)��Mg2++2OH-=Mg(OH)2������Mg(OH)2+2H+=Mg2++2H2O

��ϰ��ϵ�д�
�����Ŀ