��Ŀ����
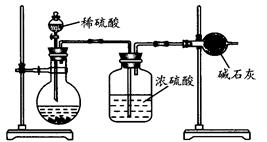
��16�֣�������ҵ��ϴ�ֲ�ʱ�����ķ�Һ��Ҫ�ɷ�ΪFe2+��H+��Cl-�������������������÷�Һ������������Ʊ�������Ϳ�ϡ� 
��1������X�Ļ�ѧʽ�� ��
��2���Ȼ�������Һ��������������ת��ΪHCl�������������ĩ���йصĻ�ѧ����ʽ����Ϊ�� ��
��3��ij����Ϳ���г�����Fe2O3�⣬������������CuO��FeO�е�һ��,�����ʵ�鷽����̽��������Ϳ����������ijɷ֡�
�� �����������
����1��������CuO
����2��������FeO
�� ����Ʒ�������֤�������裬д��ʵ�鲽�衢Ԥ������ͽ��ۡ�
��ѡ�Լ������ۡ�3mol?L-1H2SO4��0.1 mol?L-1����KMnO4��Һ��10%NaOH��Һ��10%H2O2��KSCN��Һ
| �������� | Ԥ������ͽ��� |
| ����1��ȡ������Ʒ���Թ��У� ____________________________________________ | ��Ʒȫ���ܽ⣬�õ��������Һ�� |
| ����2�� ����3�� | ___________________________ ___________________________ |
��16�֣���1�� Fe ��1�֣�д���Ʋ����֣�
��2��4FeCl2+ 4H2O+ O2  2Fe2O3 + 4HCl��3�֣�
2Fe2O3 + 4HCl��3�֣�
���� FeCl2+ 2H2O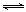 Fe(OH)2+ 2HCl��4Fe(OH)2+O2+2H2O = 4Fe(OH)3��
Fe(OH)2+ 2HCl��4Fe(OH)2+O2+2H2O = 4Fe(OH)3��
2Fe(OH)3 Fe2O3+ 3H2O��ÿʽ1�֣���3�֡�������������Ų���Ϊ�÷ֵ㣩
Fe2O3+ 3H2O��ÿʽ1�֣���3�֡�������������Ų���Ϊ�÷ֵ㣩
��3����10�֣�
��4�� 224 ��2�֣��������� Ԥ������ͽ��� ����1��ȡ������Ʒ���Թ��У�����������3mol?L��1H2SO4���������2�֣� ����2��ȡ����������Һ�ڣ������������ۣ�������ټ�������3mol?L��1H2SO4���������2�֣�
����3��ȡ��������1��Һ���Թ��У���μ���0.01 mol?L��1����KMnO4��Һ.��2�֣�
������2�Ͳ���3�IJ�����������ۿ��Ի���λ�ã� ���Թ��г��ֺ�ɫ���壬˵����������CuO��2�֣�
����Ϻ�ɫ��ȥ��˵����������FeO��2�֣�
���������������1���������̵�Ŀ�Ŀ�֪��X��Fe�����ᷴӦ�����������ӣ��ɳ�ȥ��ͬʱ�������������ʣ�
��2���Ȼ�������Һ��������������ת��ΪHCl�������������ĩ����֪�ù��̷�����������ԭ��Ӧ������Ӧ�������μӣ������൱�ڸ���ϵ���ȣ�������������Ӧ�����������ӵ�ˮ�⣬���������������������������������������������������������ֽ���������������Ըù������漰�Ļ�ѧ����ʽ�У�FeCl2+ 2H2O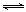 Fe(OH)2+ 2HCl��4Fe(OH)2+O2+2H2O = 4Fe(OH)3��2Fe(OH)3
Fe(OH)2+ 2HCl��4Fe(OH)2+O2+2H2O = 4Fe(OH)3��2Fe(OH)3 Fe2O3+ 3H2O��
Fe2O3+ 3H2O��
��3�����ݱ���������Ԥ������ͽ��ۣ�����1Ӧѡ���ᣬ��Ϊ������CuO����FeO���������ᣬ���Բ���1Ϊ������������3mol?L��1H2SO4���������ʱ��Һ�к���Cu2+��Fe2+��Fe3+������2��3��Ӧ�Ǽ�����Һ�е�Cu2+��Fe2+�����������Լ�������Cu2+��Ӧѡ�����ۡ�3mol?L-1H2SO4������Fe2+��Ӧ��0.1 mol?L-1����KMnO4��Һ�����岽������𰸣�
��4������635gˮ��ȫ���ա������������ա�������HCl����xmol�õ�36.5%��Ũ���ᣬ��36.5x/(635+36.5x)=36.5%�����x=10�����Կ����ձ�״���µ�HCl�������224L�ɵ�36.5%��Ũ���ᡣ
���㣺����Թ�ҵ���̵ķ�������㣬���ӵļ��飬ʵ�鷽�������

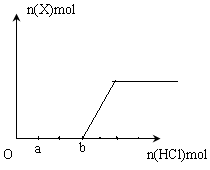
����ͬ����������NaHCO3��ĩ����һ�ݼ����������ᣬ�ʶ����ȼ���ʹ����ȫ�ֽ�
�ټ�����ͬ�������������ᣬ�����������ĵ��������Ȼ����������Ϊ
| A��2:1 | B��1:1 | C��1:2 | D��4:2 |
Al��Fe��Cu������Ҫ�Ľ���Ԫ�ء�����˵����ȷ����
| A�����߶�Ӧ���������Ϊ���������� |
| B�����ߵĵ��ʷ����ڿ����о�ֻ���������� |
C���Ʊ�AlCl3��Fe Cl3��CuCl2�����ܲ��ý���Һֱ�����ɵķ��� Cl3��CuCl2�����ܲ��ý���Һֱ�����ɵķ��� |
| D��AlCl3��FeCl3��CuCl2����������ˮ�� |
��ag Mg��Al�Ͻ���ȫ�ܽ���V1L��c1mol/L��������Һ�У�����bgH2������Ӧ�����Һ�м���V2L,c2mol/LNaOH��Һ��ǡ��ʹ�����ﵽ���ֵ���ҳ�������Ϊdg�������й�ϵʽ�������( )
| A���Ͻ��е����ʵ���Ϊ(24b-a)/9mol |
| B��d=a+17b |
C��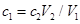 |
D���������Ӧ��ʣ����������ʵ���Ũ��Ϊ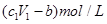 |